Breaking local symmetry—why water freezes but silica forms a glass
$ 10.00 · 4.9 (696) · In stock

Everyone knows that water freezes at 0 degrees C. Life on Earth would be vastly different if this were not so. However, water
Everyone knows that water freezes at 0 degrees C. Life on Earth would be vastly different if this were not so. However, water's cousin, silica, exhibits wayward behavior when cooled that has long puzzled scientists.
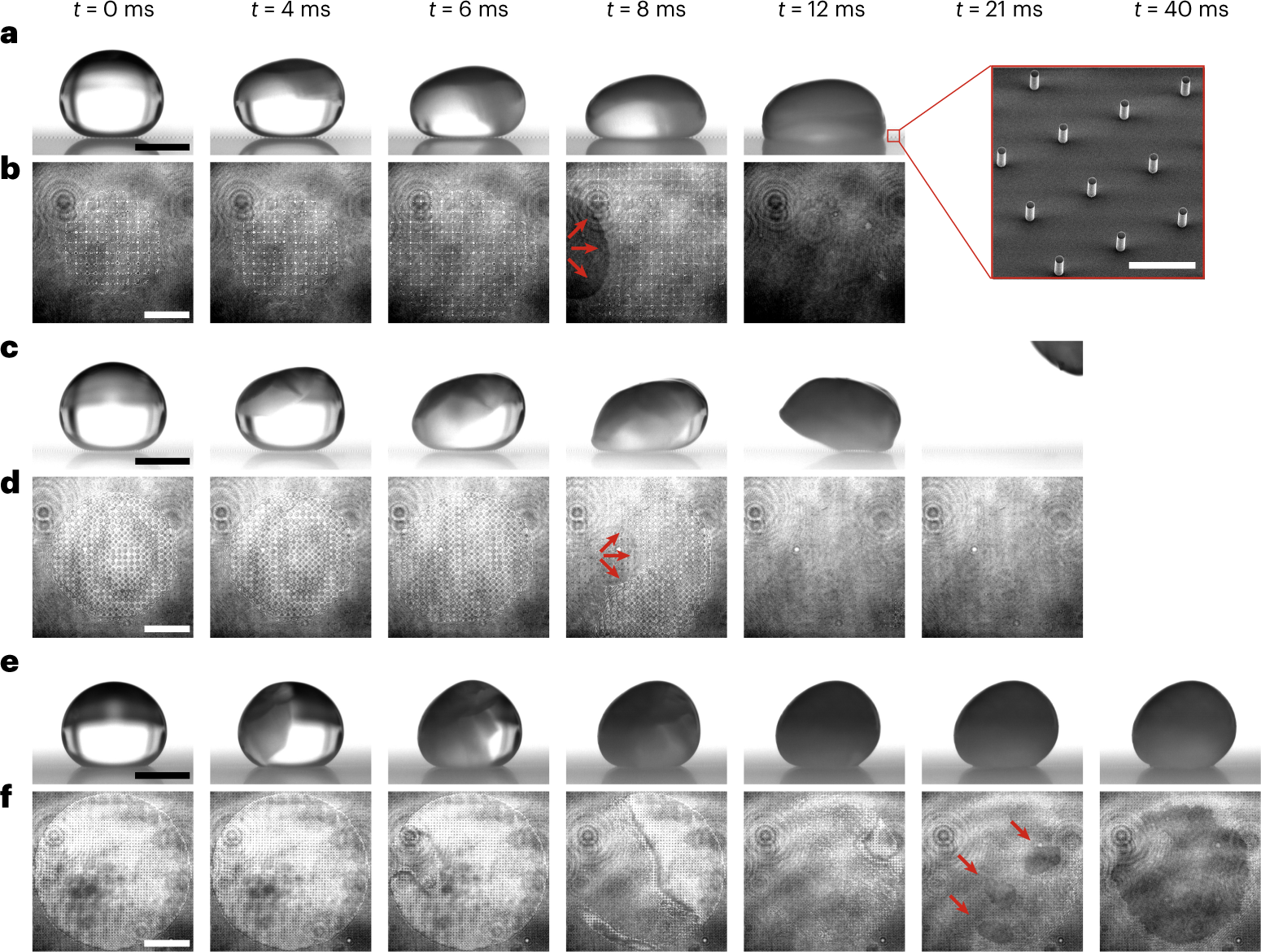
Freezing-induced wetting transitions on superhydrophobic surfaces

Various types of defects artificially produced on glass surfaces. From
Dehydration of a crystal hydrate at subglacial temperatures
Does water become less dense as it becomes colder or only when it reaches freezing temperature? - Quora
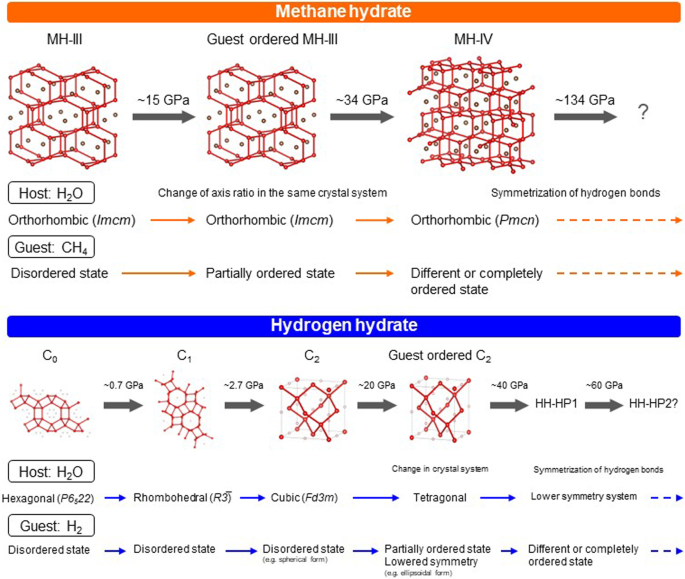
Significance of the high-pressure properties and structural evolution of gas hydrates for inferring the interior of icy bodies, Progress in Earth and Planetary Science

Understanding the strange behavior of water
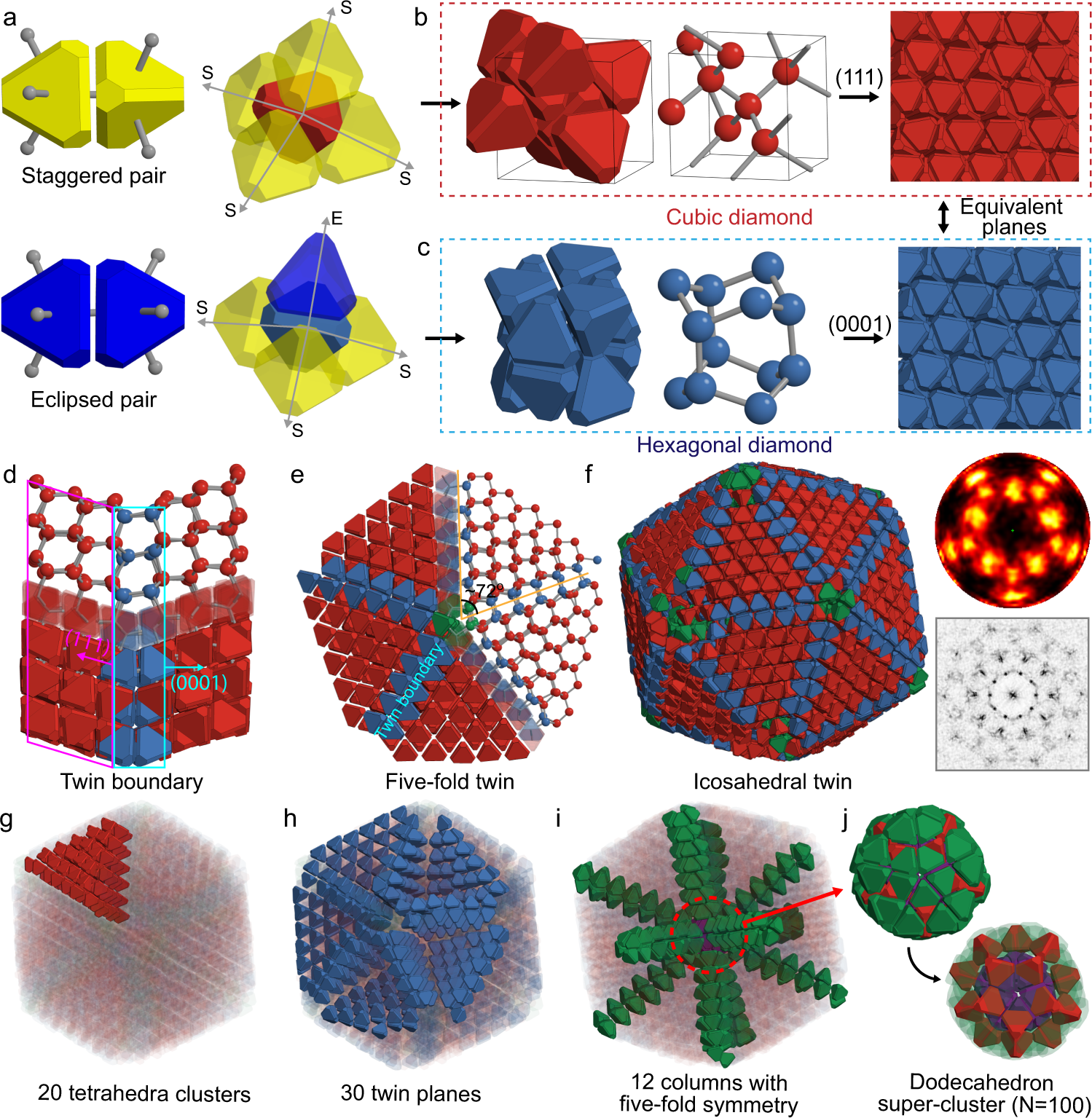
Entropically engineered formation of fivefold and icosahedral twinned clusters of colloidal shapes

Theoretical computation of the solidification front propagation

Ice Crystallization in Shear Flows The Journal of Physical Chemistry C

The viscosities of several substances plotted as functions of the

Breaking translational symmetry via polymer chain overcrowding in molecular bottlebrush crystallization

From Science Node: “The 5 fastest supercomputers in the world

Freezing life within refractory, amorphous silicon dioxide
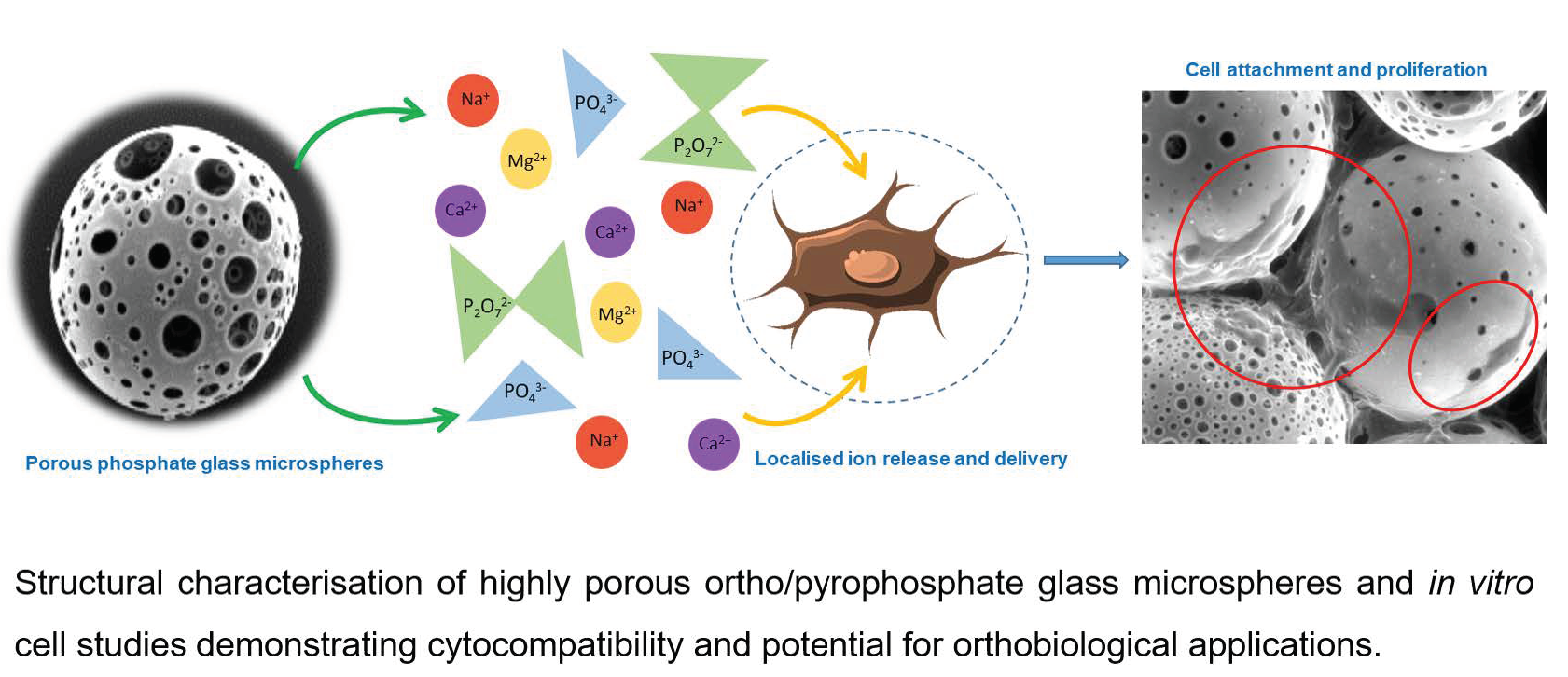
Bioengineering, Free Full-Text

Scientists offer designer 'big atoms' on demand