32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
$ 19.99 · 4.9 (228) · In stock
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32- 80 g of h2 is reacted with 80 g of o2 to form water- find out the mass of water obtained-which substance is the limiting reagent

Aqueous Transformation of a Metal Diformate to a Metal Dihydride Carbonyl Complex Accompanied by H2 Evolution from the Formato Ligands

Question Video: Calculating the Mass of Water Produced Given the Masses of Oxygen and Hydrogen

41. 8g H2 and 32 g 0, is allowed to react to form water then which of the following statement is correct (1) O, is limiting reagent (2) O, is reagent in

Gas Stoichiometry - Chemistry

Percent Yield Formula, How to Calculate Yield - Lesson

80 g of H_2 is reacted with 80 g of O_2 to form water. Find out the mass of water obtained . Which substance is the limiting reagent ?
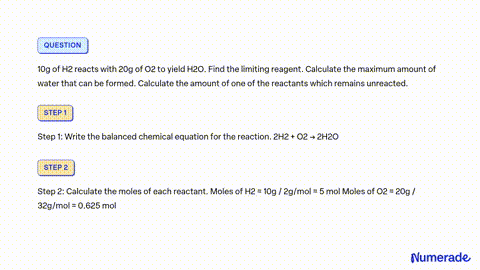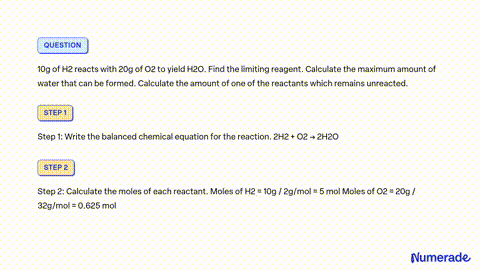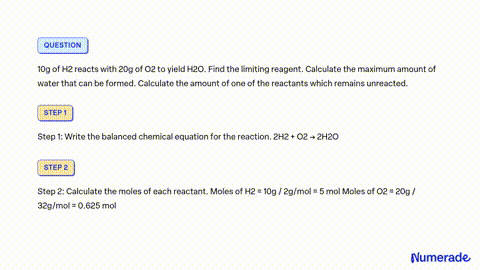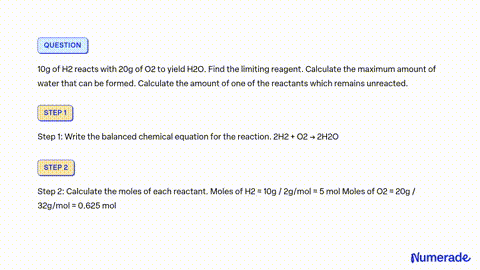
SOLVED: 80 g of H2 is reacted with 80 g of O2 to form water. Find out the mass of water obtained. Which substance is the limiting reagent?

Chapter 3 Chemical Reactions and Reaction Stoichiometry - ppt download

In the reaction H2 + O2 =H20. If 6g of H, combines with 64g of Oz. Find mass of Excess reagent left? 32 g 48 g 16 g None of these

Formic acid as renewable reagent and product in biomass upgrading - Tetrahedron Green Chem
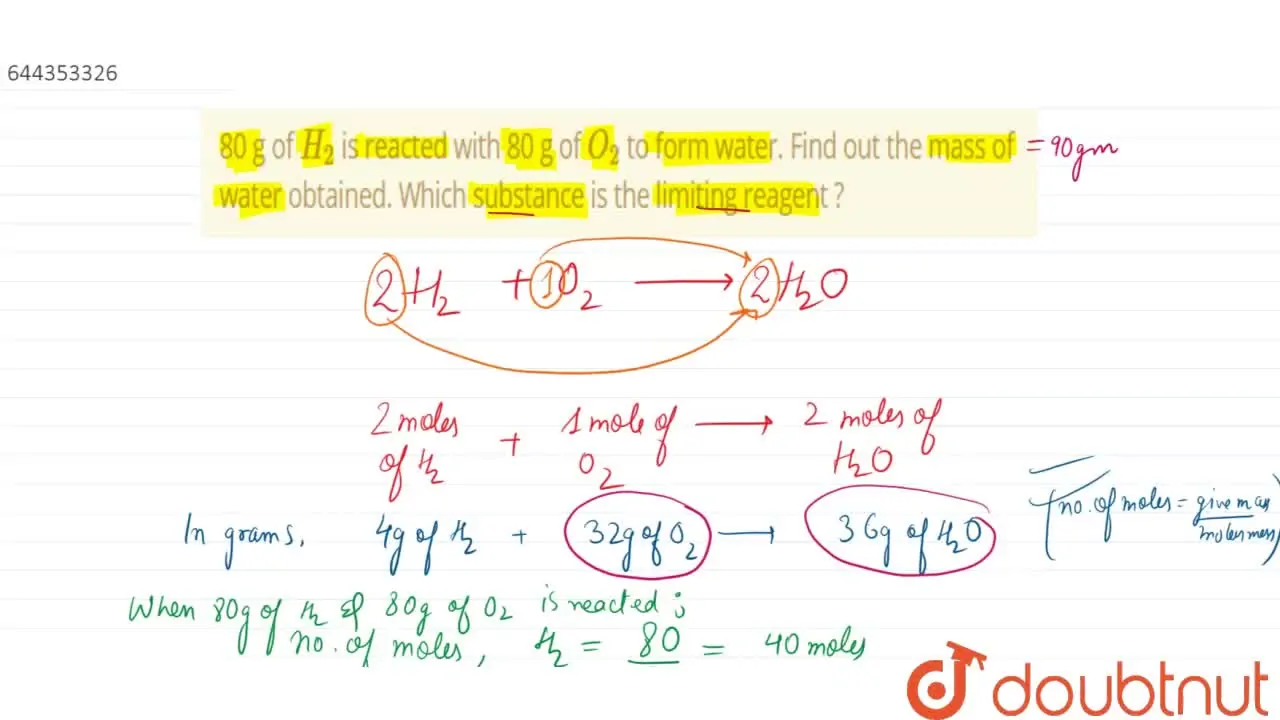
80 g of H(2) is reacted with 80 g of O(2) to form water. Find out the
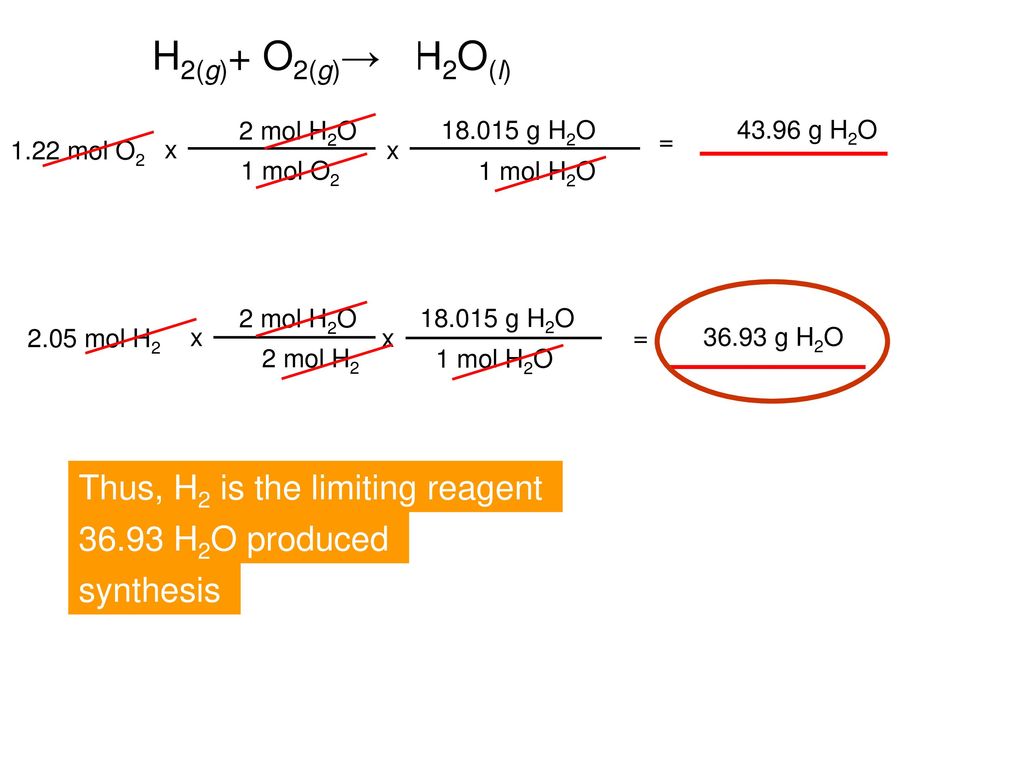
2H2(g)+ O2(g)→ 2H2O(l) Thus, H2 is the limiting reagent - ppt download

GC 1 Flashcards