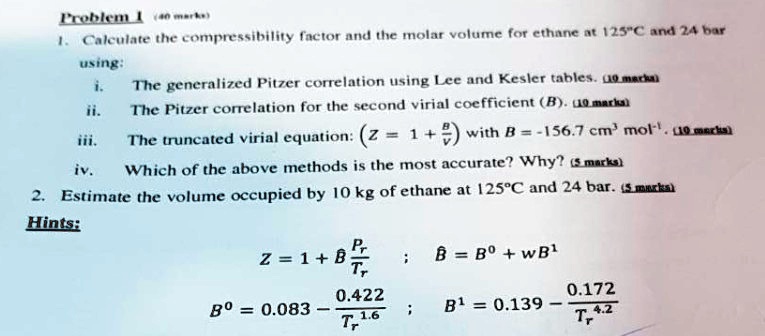
SOLVED: Problem 1: Calculate the compressibility factor and the molar volume for ethane at 125°C and 24 bar using: i. The generalized Pitzer correlation using Lee and Kesler tables. ii. The Pitzer
$ 11.00 · 4.6 (671) · In stock
VIDEO ANSWER: There is a chance that the pressure will be 300 bar and the temperature will be zero degree centigrade, so it will be a temperature of 3700 degrees. The T is equal to the number of moles. R is the universal gas constant, and the ideal
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.

A tank contains a mixture of 70% ethane and 30% nitrogen $(N

Study of Liquid−Liquid and Liquid−Liquid−Vapor Equilibria for Crude Oil Mixtures with Carbon Dioxide and Methane Using Short- Wave Infrared Imaging: Experimental and Thermodynamic Modeling
Solved Use the Pitzer correlation in Z (using Lee/Kester

Binary interaction parameters for nonpolar systems with cubic equations of state: a theoretical approach 1. CO2/hydrocarbons using SRK equation of state

Lecture 12 & 13.pdf - Generalized correlations for gases Pitzer correlations for the compressibility factor: Z Z 0 Z 1 o Z0 = F0 Tr Pr o Simple
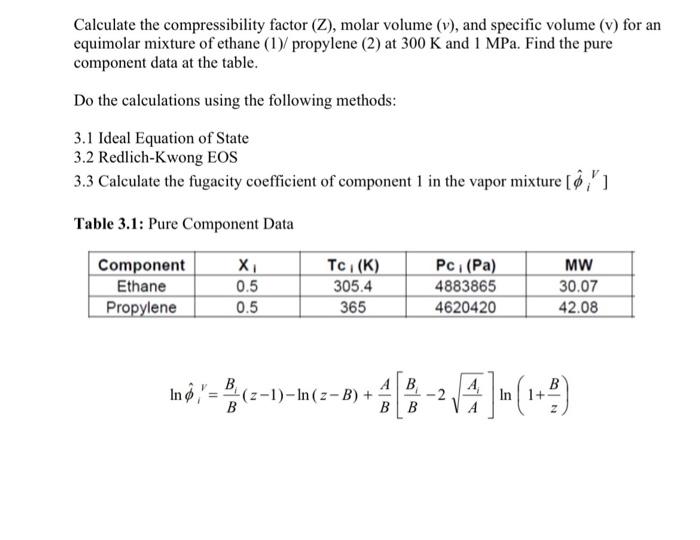
Solved Calculate the compressibility factor (Z), molar
Solved uestion 2 20 poin Q2: [20] pts Calculate the values

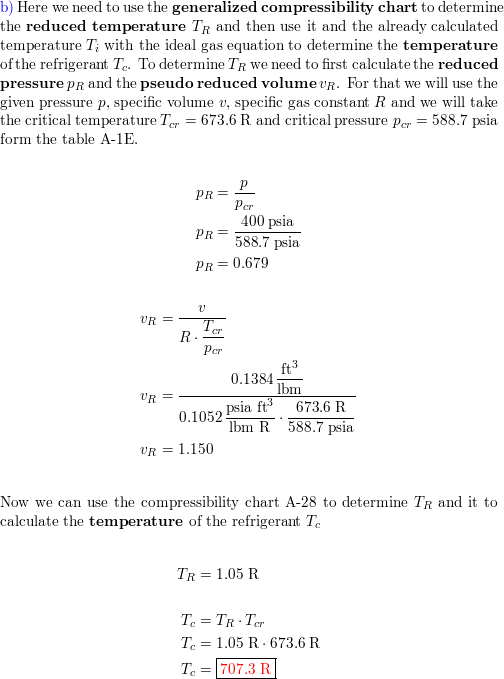
Refrigerant-134a at 400 psia has a specific volume of $0.138
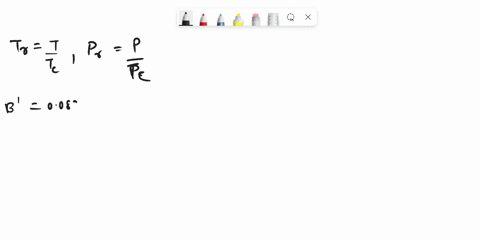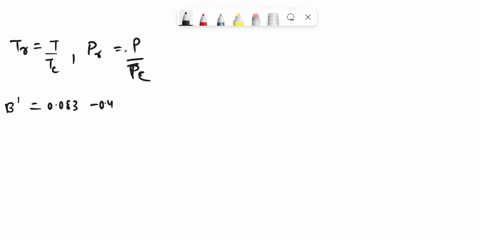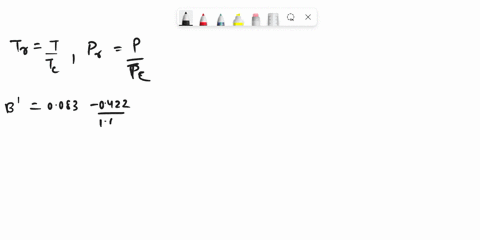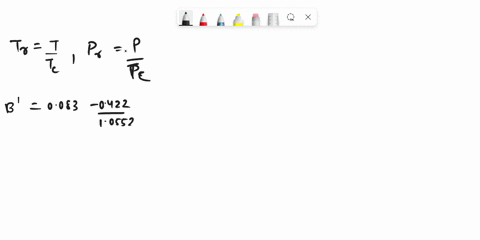
SOLVED: Problem 3 (35 marks): Calculate the compressibility factor Z and specific volume V cm/mole for ethane at 47.5°C and 25 bar by the following equations: 1. Ideal gas equation - 5
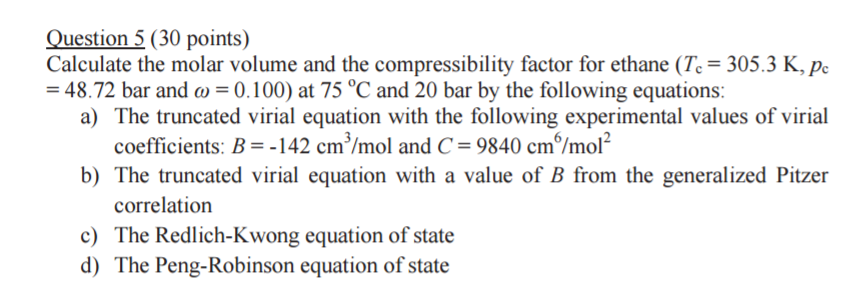
Calculate the molar volume and the compressibility

Calculate Z and V for ethylene at 25°C and 12 bar by the following equations: (a) The truncated virial