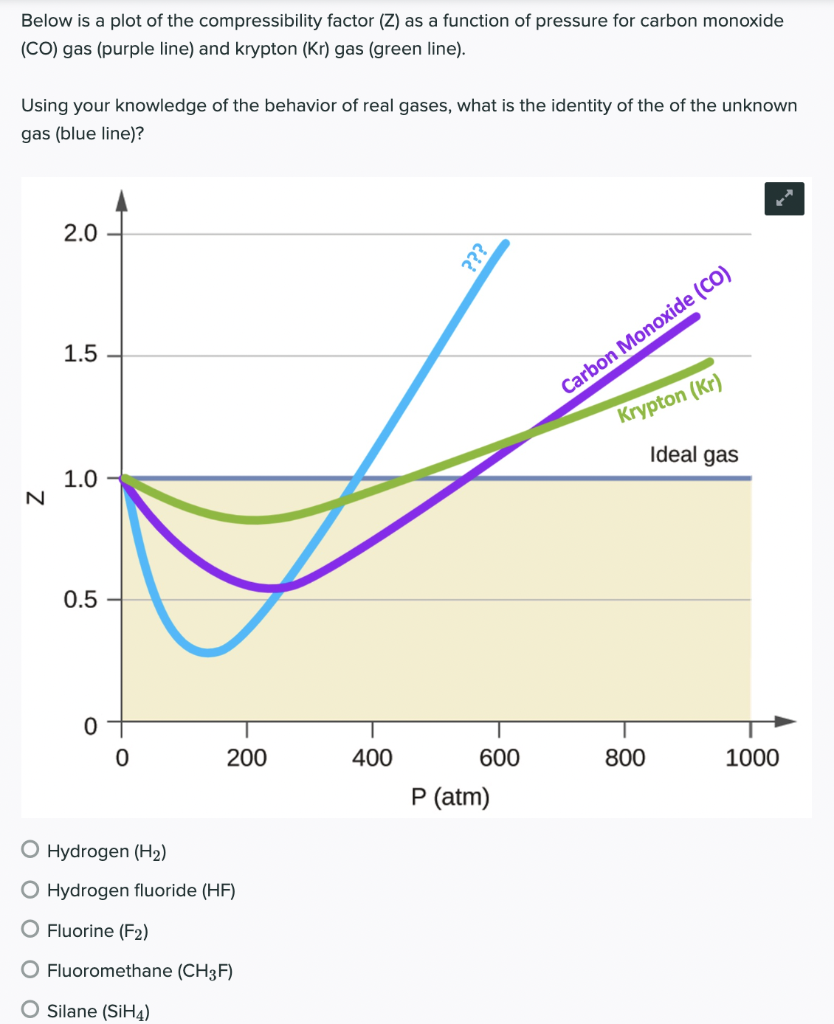
Solved Below is a plot of the compressibility factor (Z) as
$ 19.99 · 4.8 (209) · In stock
Real Gases vs Ideal Gases & the Compressibility Factor
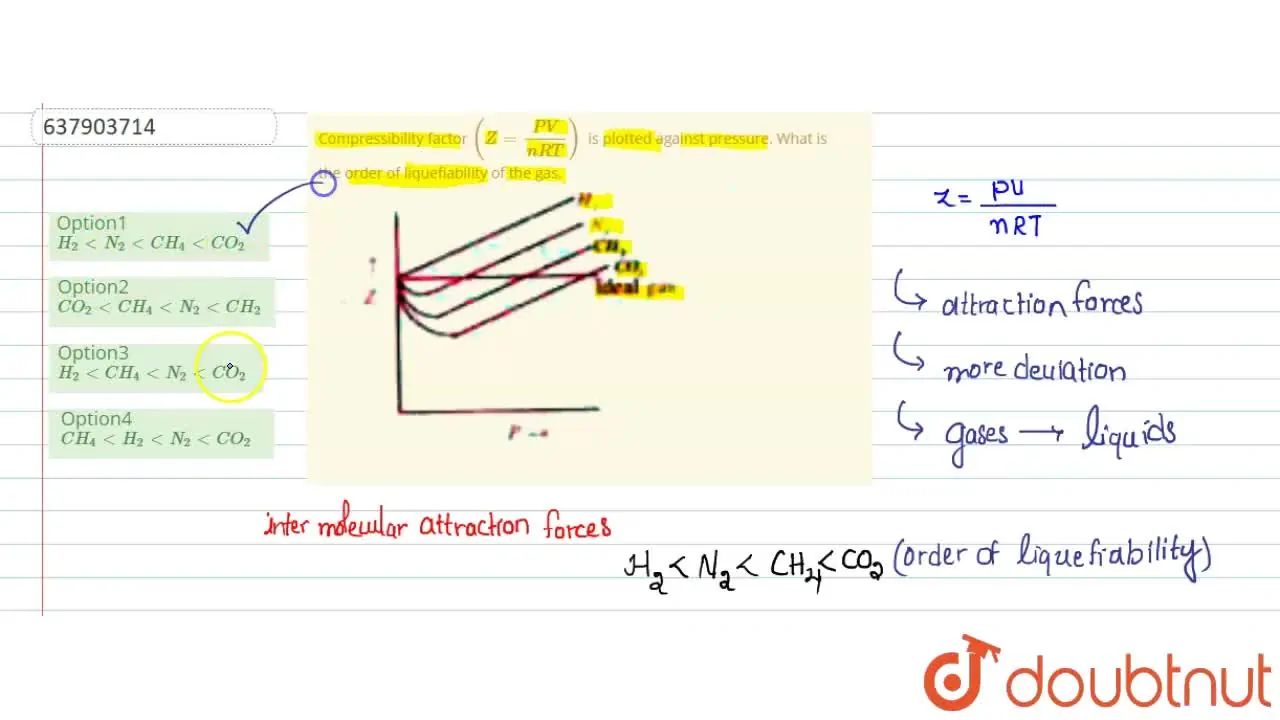
Telugu] Compressibility factor (Z = (PV)/(nRT)) is plotted against p

The given graph represents the variation of compressibility factor Z vs P for three gases A, B and C.Identify the incorrect statements.
Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
For 1 mole of gas, the plot of pV vs p is the pressure and V What is
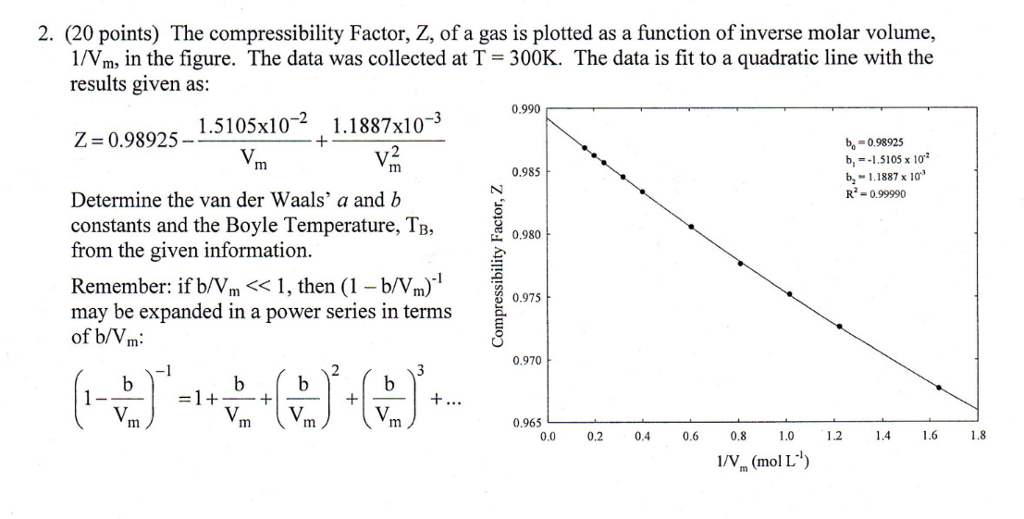
Solved 2. (20 points) The compressibility Factor, Z, of a

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

The role of the compressibility factor Z in describing the volumetric behavior of gases
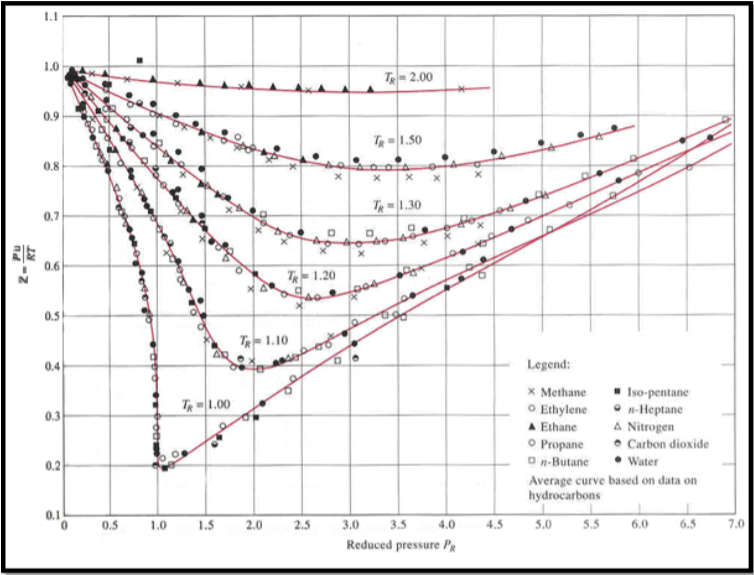
Solved Use the plot of compression factor (Z) vs reduced

Compressibility factor for methane.

The graph of compressibility factor (Z) v/s P 1 mol of a real gas is shown in following diagram. The graph is plotted 273 K temperature. If slope of graph very high

The given graph represents the variation of compressibility factor Z vs P for three gases A, B and C.Identify the incorrect statements.

