117. Compressibility factor H, behaving as rea gas is 1) 1 RTV 3) 1+- RT 4) (1-a) 18. If V is the observed molor unlum
$ 19.00 · 4.6 (229) · In stock

Click here:point_up_2:to get an answer to your question :writing_hand:117 compressibility factor for h behaving as reagas is1 1rtv31rt41a18 if v is the observed
Click here👆to get an answer to your question ✍️ 117- Compressibility factor H- behaving as rea gas is 1- 1 RTV 3- 1- RT 4- -1-a- 18- If V is the observed molor unlum
Solved 1 1 point If the root mean square speed of a gas

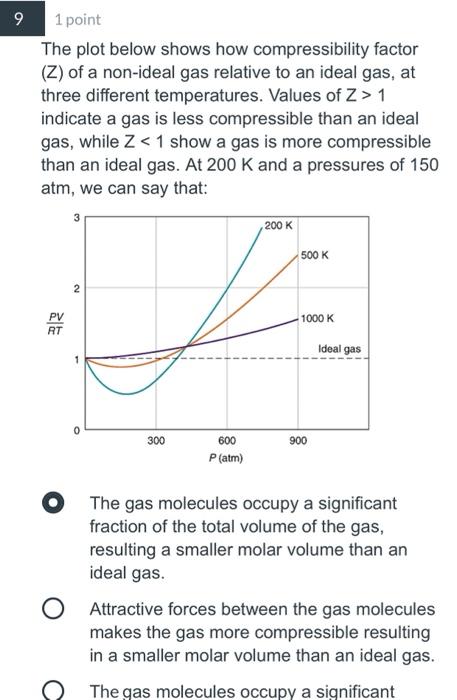
Explain how the compression factor varies with pressure and

A comprehensive survey of research towards AI-enabled unmanned aerial systems in pre-, active-, and post-wildfire management
Gasdynamics PDF, PDF, Mach Number

Non-Ideal Gas Behavior Chemistry: Atoms First

Compressibility factor (z): real gases deviate from ideal behav-Turito

Solved Data: Ideal gas constant R 8.314 J mol-1 K-1 1(a)

Systems and Appendices, Long range aerospace manufacturing developments. Volume II. Base metal forms, forming, material removal, and joining. Report

2. U 0.52, 0.68, 0.74 At low pressure, the comprensibility factor is given as (1) - RTV Pb RT 12 12 Photo (3) 1+ TV Pb RT 3. 10 mole of an ideal gas 27°C ernands

171. CH4 gas is behaving non-ideally. Compressibility factor gas is 1.5 2 atm, 400 K. Calculate molar volume gas: [Given : R=0.08 Litre-atm, K-mole (1) 24 litre (2) 16 litre (3) 48 litre (4) 8 litre
Solved QUESTION 2. Using the equation below, calculate the
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect

The compressiblity factor Z for 1 mole of a real gas at low pressure can be written as