The compressibility factor Z a low-pressure range of all gases except hydrogen is:Z=(1+ displaystylefrac{a}{V_{m}RT})Z =(1-displaystylefrac{a}{V_{m}RT})Z=(1+displaystylefrac{Pb}{RT})Z = ( 1 - displaystylefrac{Pb}{RT})
$ 9.50 · 5 (553) · In stock

Click here:point_up_2:to get an answer to your question :writing_hand:the compressibility factor z at a lowpressure range of all gases except hydrogen is
Click here👆to get an answer to your question ✍️ The compressibility factor Z a low-pressure range of all gases except hydrogen is-Z-1- displaystylefrac-a-V-m-RT-Z-1-displaystylefrac-a-V-m-RT-Z-1-displaystylefrac-Pb-RT-Z - - 1 - displaystylefrac-Pb-RT-
The van der Waals equation for real gases is -P-aVm2-Vm-x2212-b-RT

Explain how the compression factor varies with pressure and

Except H(2) and He, the compressibility factor Z(=(PV)/(nRT))lt1 for a

Non-Ideal Gas Behavior

Non-Ideal Gas Behavior Chemistry: Atoms First

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange
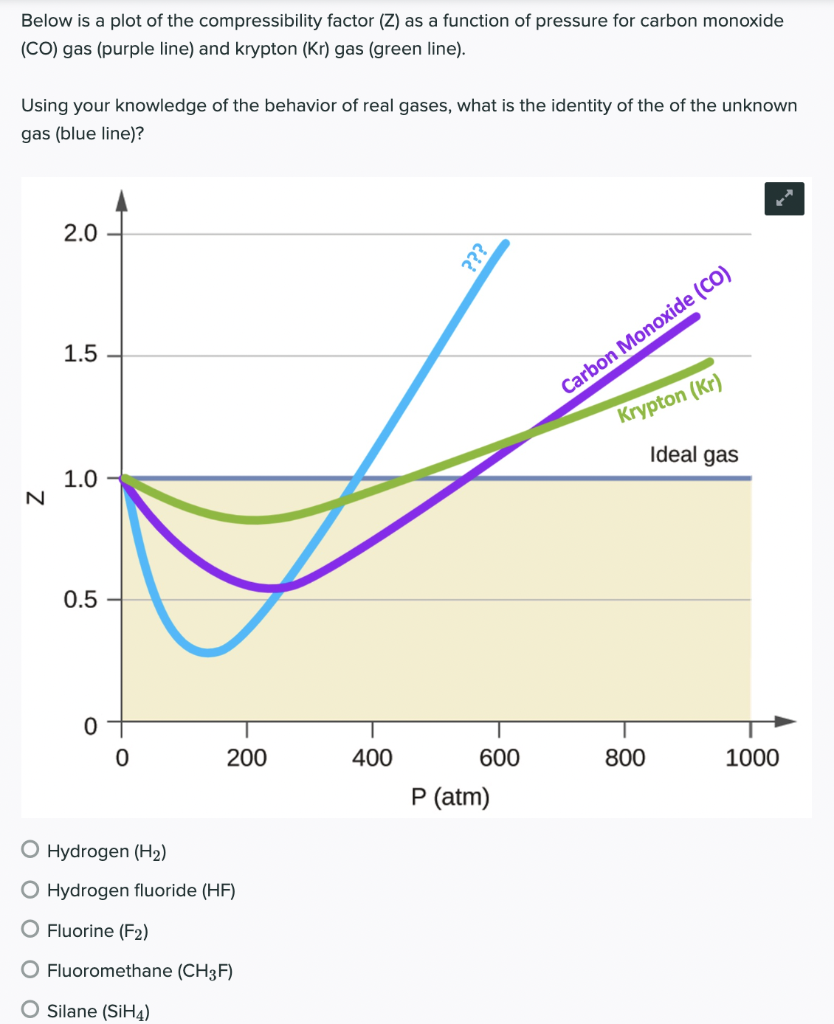
Solved Below is a plot of the compressibility factor (Z) as

Numerical simulation of fractured horizontal well considering

Gas Compressibility - an overview
Solved The plot below shows how compressibility factor (Z)