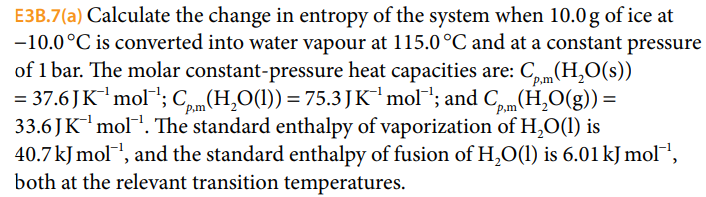
The entropy change for the conversion of 36 g water to vapour at
$ 11.50 · 4.6 (518) · In stock
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is (Enthalpy of vaporization for water is 40.63 kJ mol–1)
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is -Enthalpy of vaporization for water is 40-63 kJ mol-1
calculate the change in entropy for the conversion of one mole of liquid water to - Myschool

to insulated co 9.0 g ice O'C is mixed with 36 g of water 50°C in a thermally data, answer the question that follow ? Comprehension Q.8 Th rea 8 Final temperature
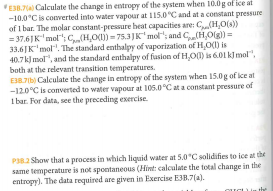
E Calculate the change in entropy of the system when
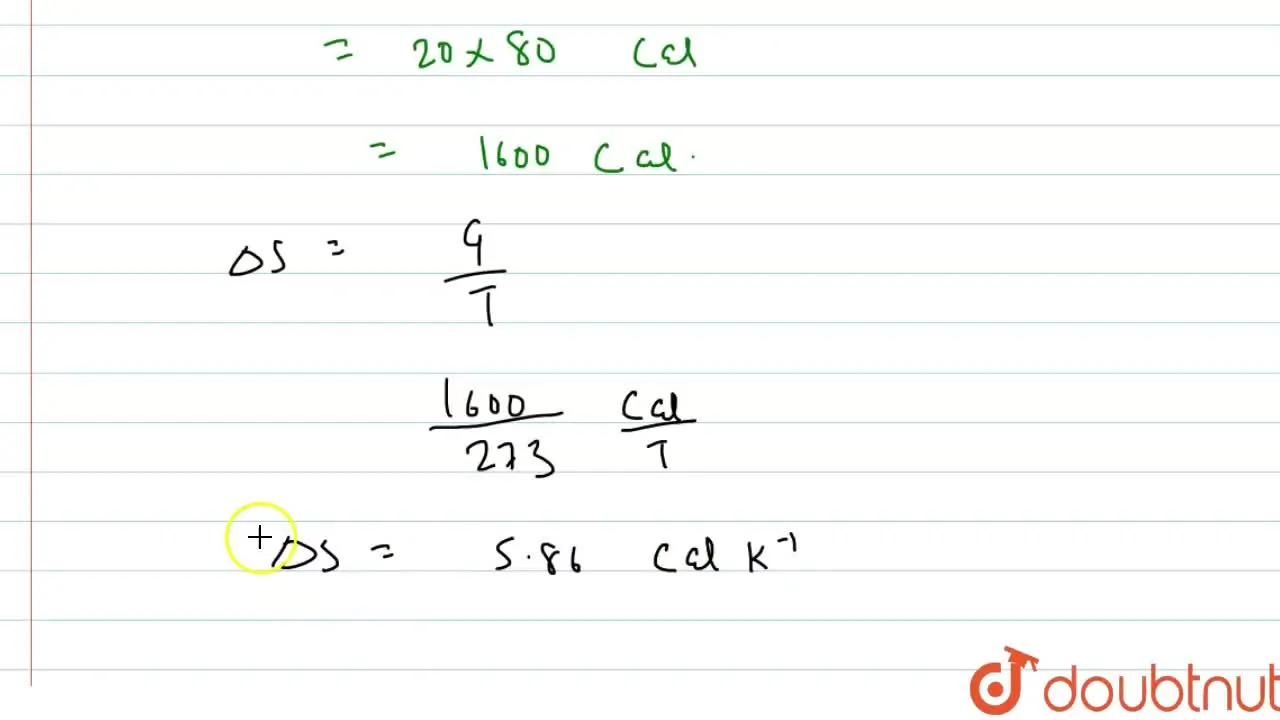
Calculate the entropy change when 20.0 g of ice changes to liquid wate

Energies, Free Full-Text
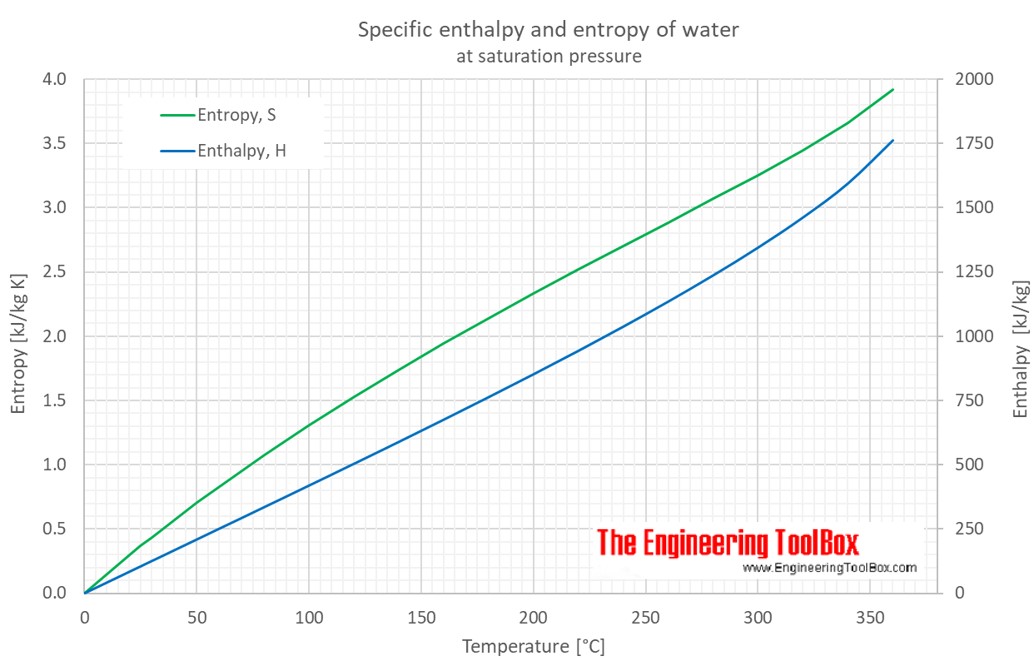
Water - Enthalpy and Entropy vs. Temperature

The concept of dynamic evaporation enabled by reconfigurable Fe3O4@G
Solved E3B.7(a) Calculate the change in entropy of the

At 373 K, the entropy change for the transition of liquid water to ste

What is the correct method to convert volumetric flow rate of mixture to mass flow rate ? : r/thermodynamics