What is the compressibility factor (Z) for 0.02 mole of a van der
$ 21.99 · 4.9 (136) · In stock

Vapor-liquid equilibrium: short note

Energies, Free Full-Text
Which gases behave least like ideal gases? - Quora

SOLVED: One mole of a gas occupies 0.5 L at 27°C. The compressibility factor of the gas at this temperature is 0.8. If b = 0.04 mol^-1, what is the value of

Influences of Hydraulic Fracturing on Fluid Flow and Mineralization at the Vein-Type Tungsten Deposits in Southern China

Free Energies of Molecular Bound States in Lipid Bilayers: Lethal Concentrations of Antimicrobial Peptides: Biophysical Journal

Write the expression for the compressibility factor (Z) for one mole of a gas. Write the value of Z for an
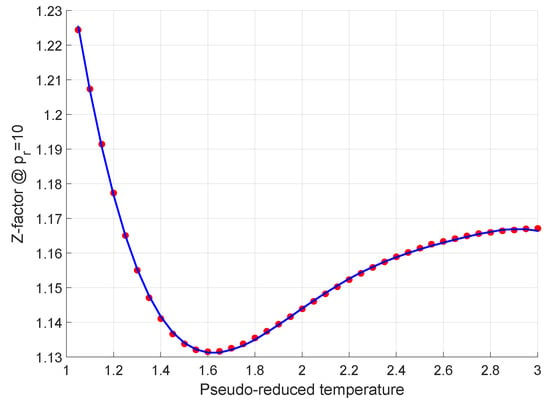
Energies, Free Full-Text

Modelling two-phase Z factor of gas condensate reservoirs: Application of Artificial Intelligence (AI) - ScienceDirect
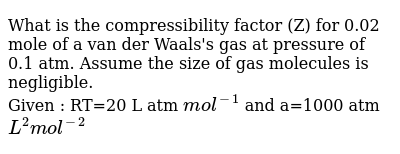
What is the compressibility factor (Z) for 0.02 mole of a van der Waal

What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0
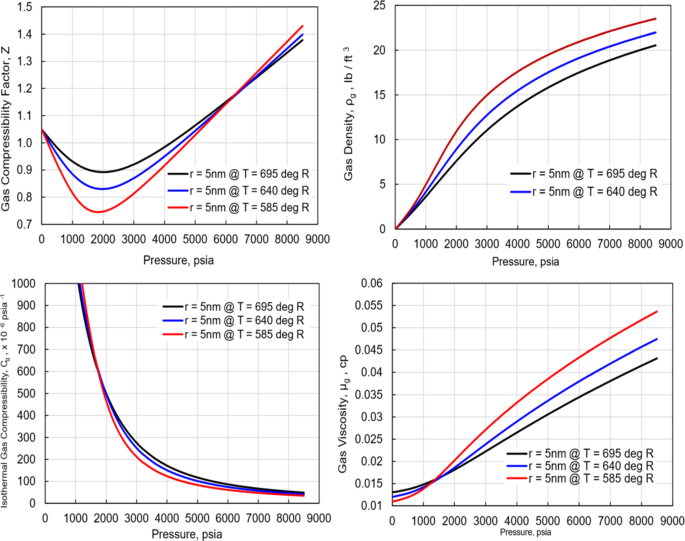
Investigation of the Properties of Hydrocarbon Natural Gases Under Confinement in Tight Reservoirs Due to Critical Properties Shift

63. What is the compressibility factor (2) 0.02 mole of a van der Waals' gas pressure of 0.1 atm. Assume the size of gas molecules is negligible. Given : RT = 20
