Gas compressibility factor Z: Ideal gas vs Real gas
$ 12.99 · 4.8 (362) · In stock

Gas compressibility factor, Z, and Gas compressibility are not the same. Gas compressibility factor Z is the ratio of the gas volume at a given temperature and pressure to the volume the gas would occupy if it were an ideal gas at the same temperature and pressure.

Real Gases Introductory Chemistry – 1st Canadian Edition

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

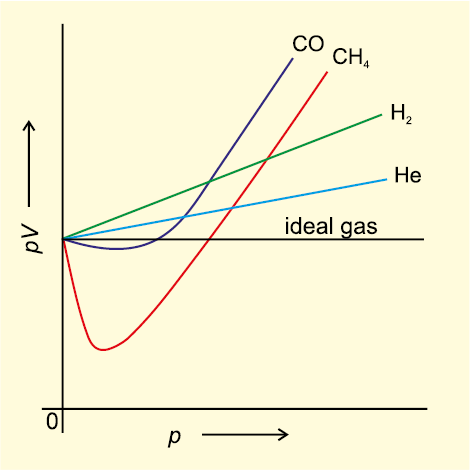
Compressibility factor for methane.
7 Tips on Compressor Design (For Students and Practicing Engineers)

gas laws - Graph of compressibility factor vs pressure when real gas is assigned Z=1 - Chemistry Stack Exchange
What is the main difference between Process Plant Piping Engineering and Pipeline Engineering.?

New explicit correlation for the compressibility factor of natural gas: linearized z-factor isotherms

COMPRESSIBILITY FACTOR
Understanding Gulf Coast Netback Prices

4.2: Real Gases (Deviations From Ideal Behavior) - Chemistry LibreTexts
Sections

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt
Real Gas Lab, PDF, Gases

Compressibility Factor - an overview



