What is compressibility factor? What is its value for ideal gas
$ 14.50 · 4.6 (550) · In stock

What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

Energies, Free Full-Text
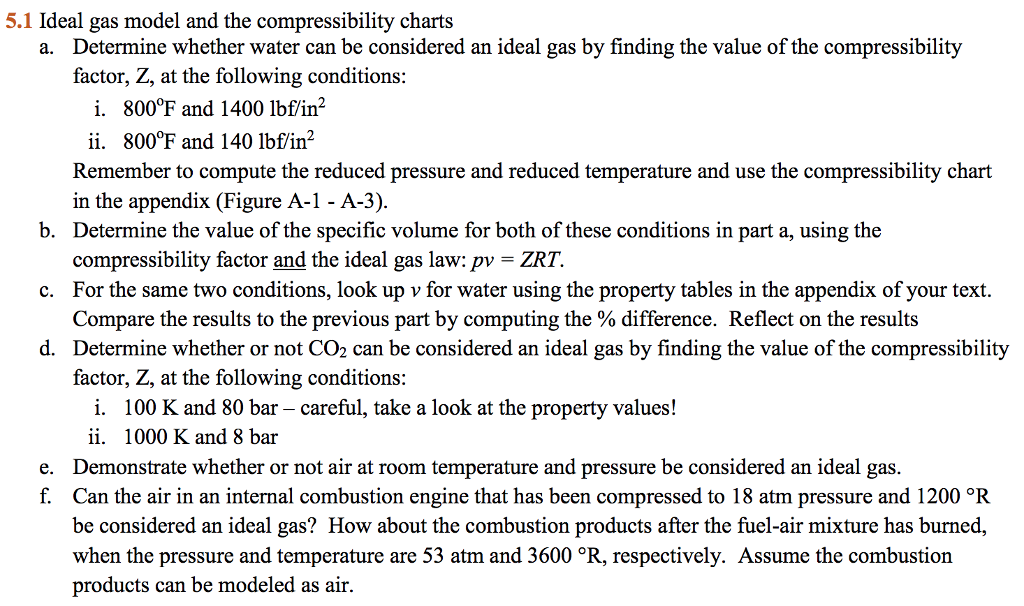
Solved 5.1 Ideal gas model and the compressibility charts
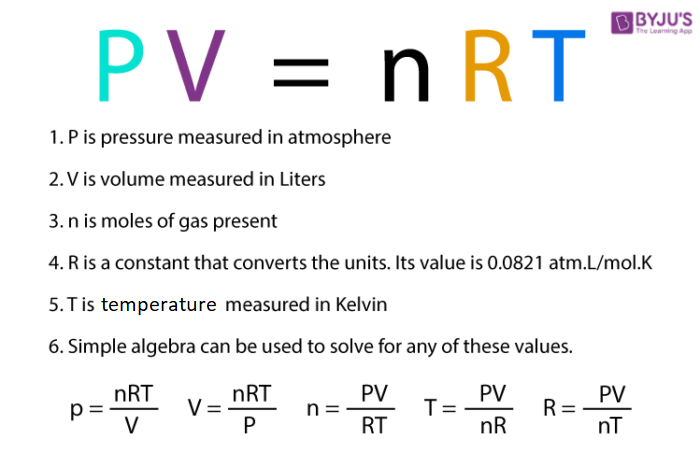
Ideal Gas Law Equation Compressibility Of Natural Gas - Chemistry

The compressibility factor for an ideal gas is ?

If compressibility factor Gas A, Gas B, Gas C and Gas D1.6,0.8,0.4,1.8 respectively than (i) Nature of gas (ii) Increasing order of force of attraction b/w the molecules (iii) which one gas

The given graph represents the variation of Z (compressibility factor = \[\dfrac{{PV}}{{nRT}}\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.

Procedure calculates base gas compressibility factors

Thermodynamics - 3-7 Ideal Gas Equation with compressibility factor