Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt
$ 25.99 · 4.8 (92) · In stock


Compressibility factor, Z of a gas is given as Z = pV / nRTi What is the value of Z for an ideal gas?ii For real gas what will be the effect
Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT } (i) What is the value of Z an ideal gas?(ii) For real gas what will be

ANSWERED] Q 32 Compressibility factor Z of a gas is given as Z pV nRT - Kunduz

Solved We begin by showing that the compressibility factor

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics
Sections

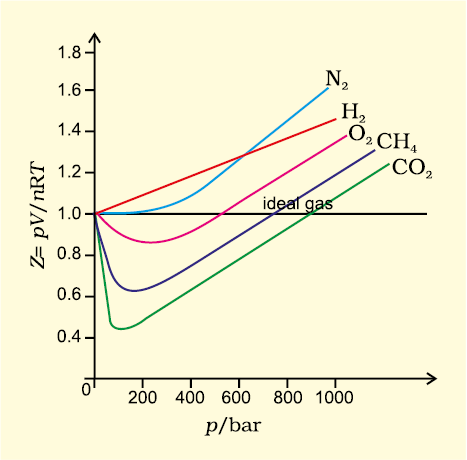
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

Chapter 03 thermo

Chemistry!!! Not Mystery : Do Real Gases Behave Ideally?
Ideal Gases & Real Gases, PDF, Gases

Compressibility Factor of Gas Overview, Equation & Chart
