Just a few neoantigens may be enough for T cells to control prostate cancer
$ 22.50 · 4.8 (669) · In stock
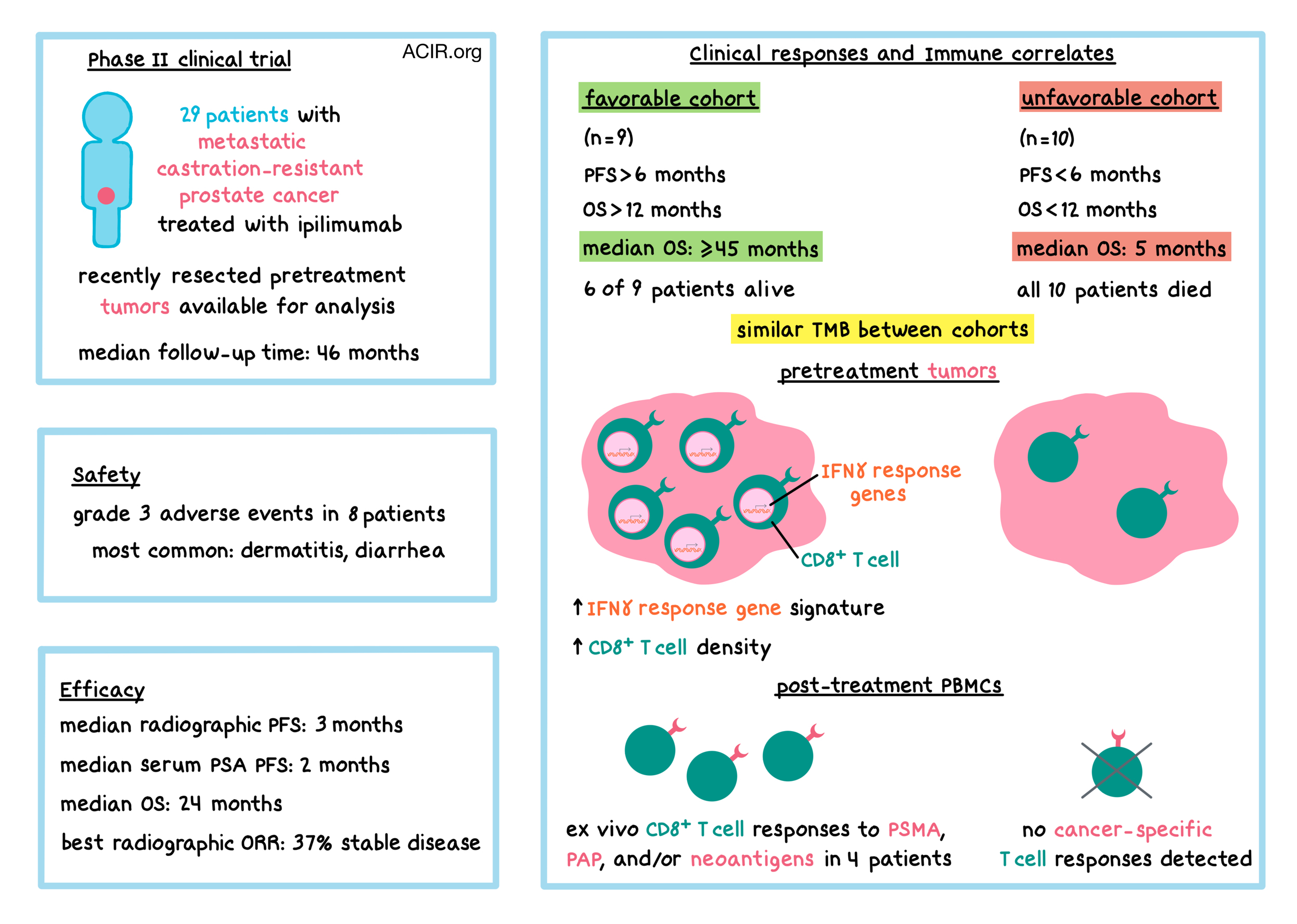
In a phase II clinical trial, 29 patients with metastatic castration-resistant prostate cancer were treated with ipilimumab after tumor resection. Median radiographic PFS was 3 months, median clinical PFS was 2 months, and median OS was 24 months. Best ORR was stable disease in 37% of patients. In the “favorable” cohort (PFS>6 months, median OS of 45 months), pretreatment tumors had increased CD8+ T cell density and IFNγ response gene signature compared with the “unfavorable” cohort (PFS<6 months, median OS of 5 months), while TMB was similar between cohorts. In post-treatment PBMCs, CD8+ T cell responses to PSMA, PAP, and/or neoantigens were found in 4 patients, all of which were in the favorable cohort.
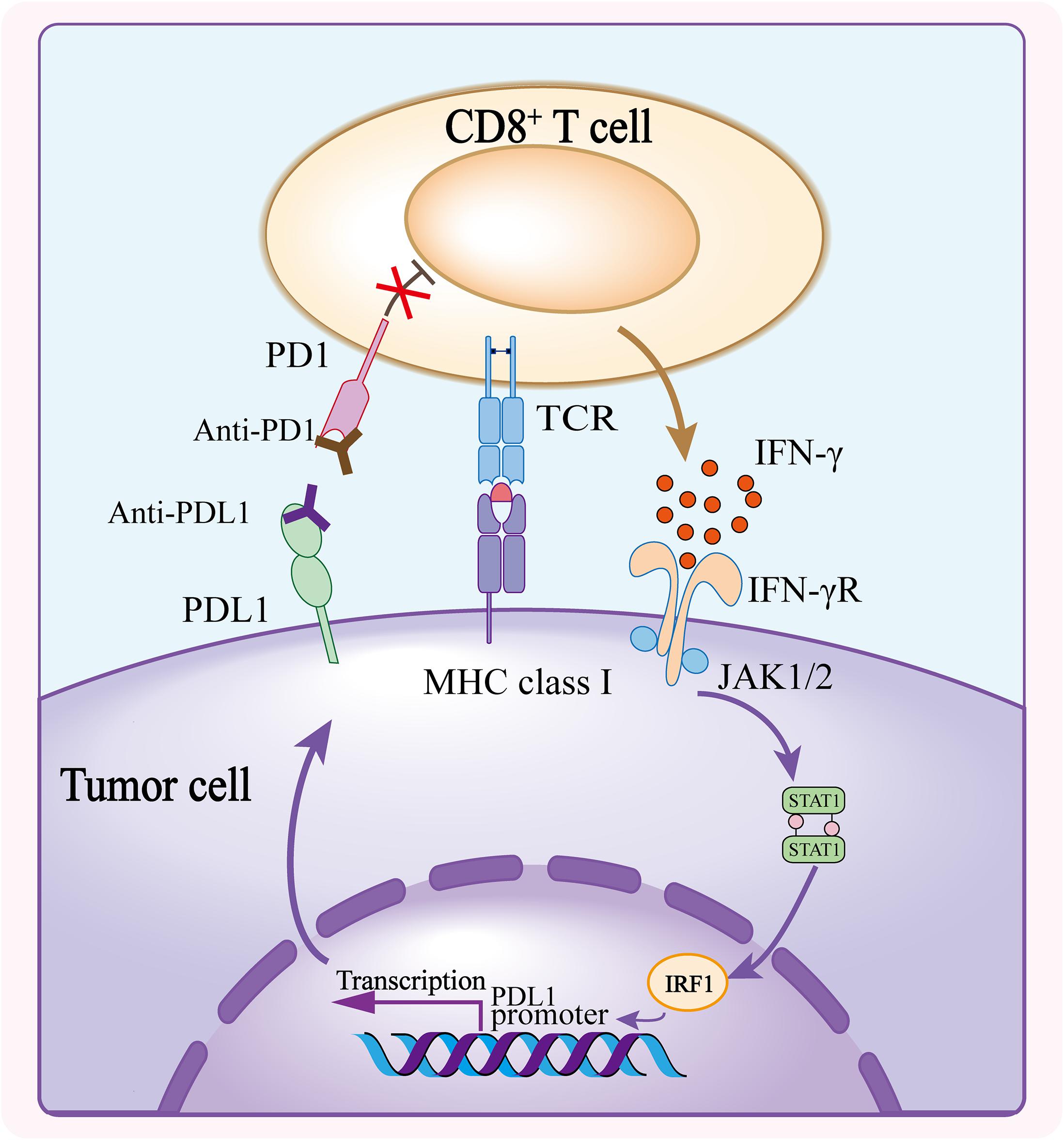
Frontiers Resistance Mechanisms of Anti-PD1/PDL1 Therapy in

Cancer vaccines: Building a bridge over troubled waters. - Abstract - Europe PMC

Immunotherapy mechanisms in prostate cancer. a Sipuleucel-T

The role of neoantigen in immune checkpoint blockade therapy, Experimental Hematology & Oncology

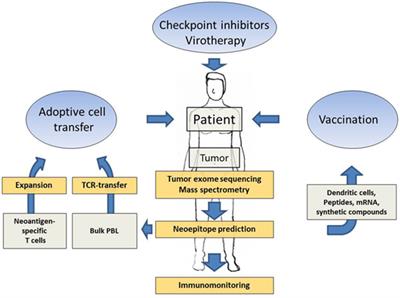
Targeting neoantigens to augment antitumour immunity
Uro, Free Full-Text
Frontiers Neoantigen Targeting—Dawn of a New Era in Cancer
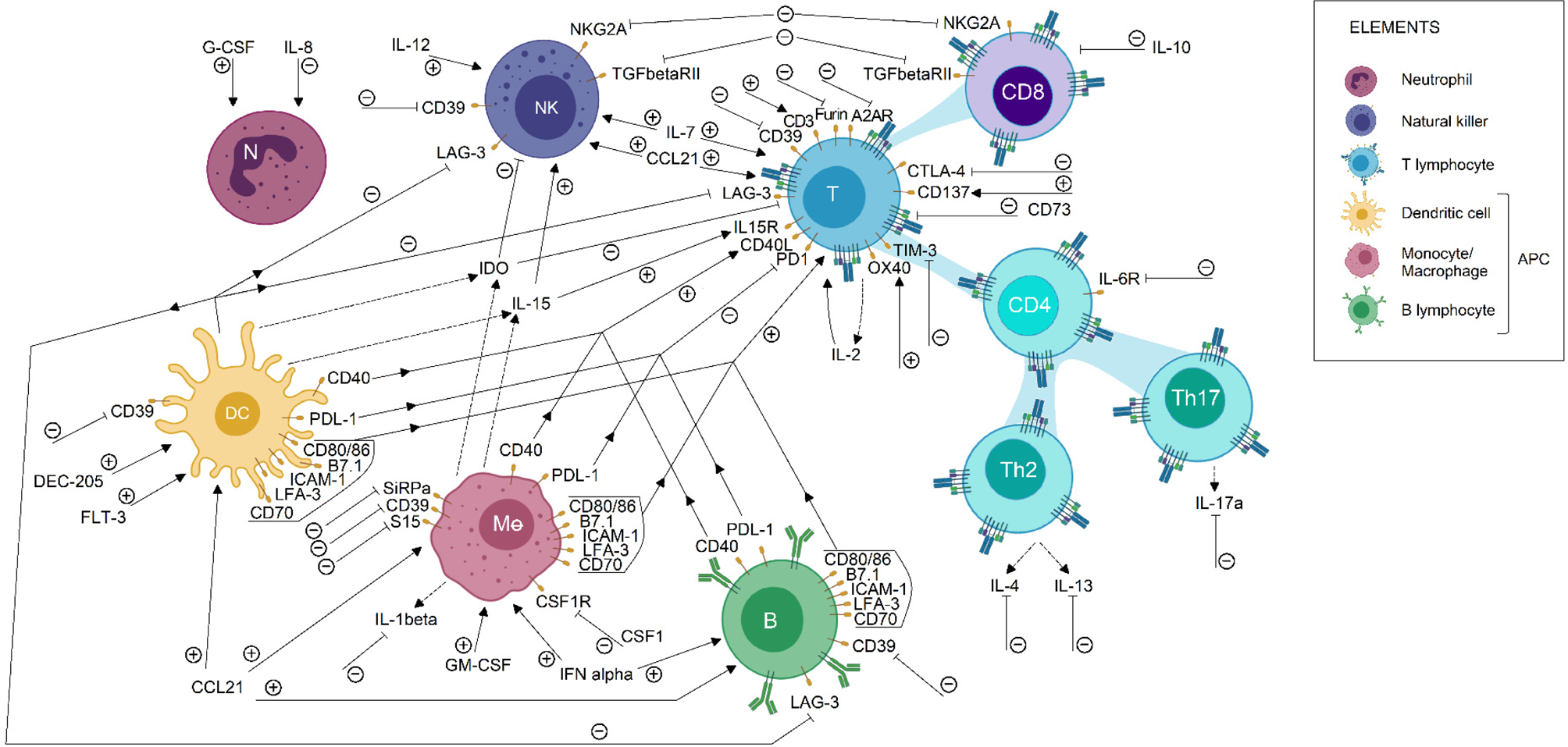
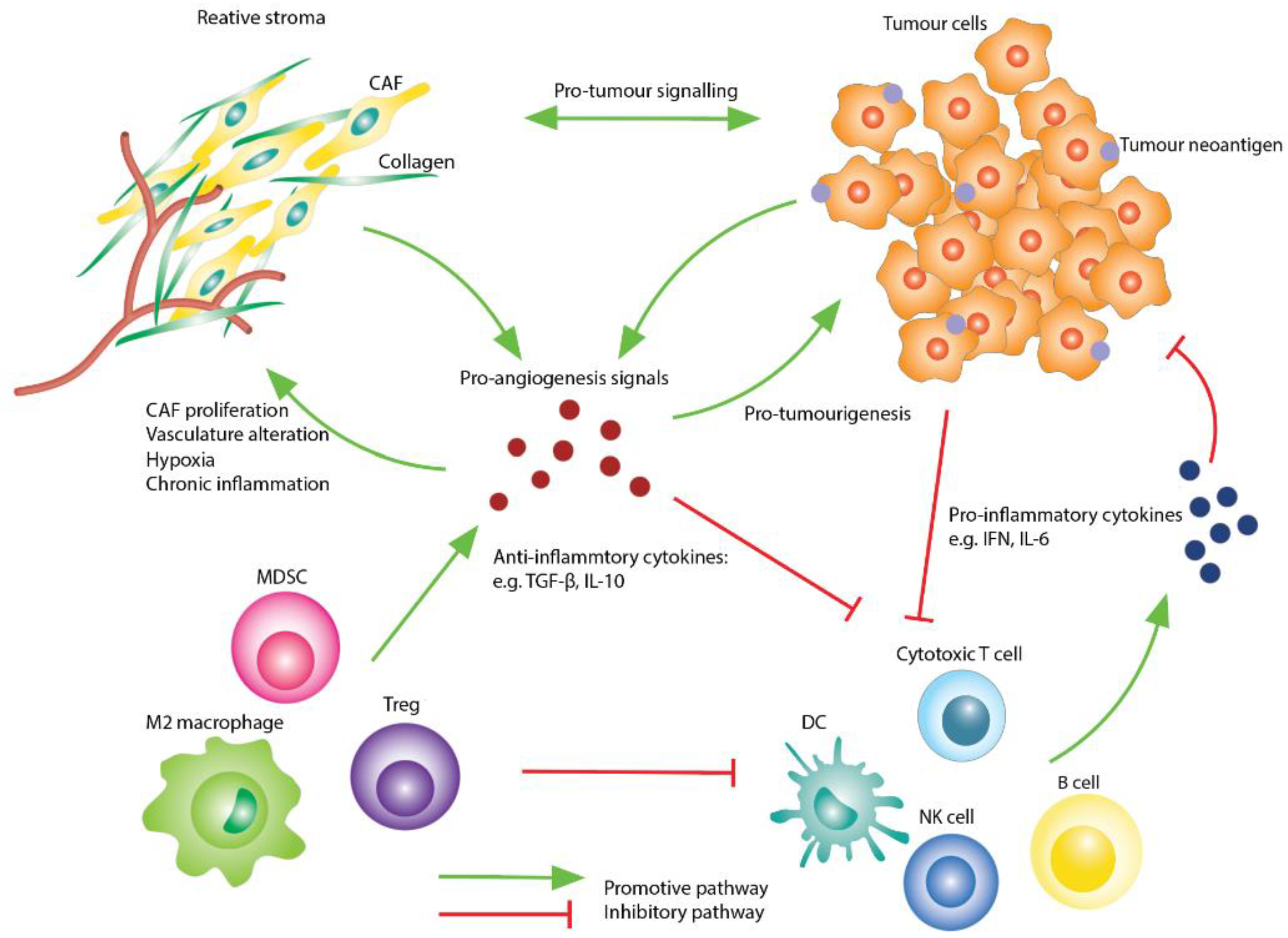
Biological bases of cancer immunotherapy

Harnessing the potential of CAR-T cell therapy: progress