Solved The compressibility factor, Z, can be thought of as a
$ 19.99 · 4.5 (425) · In stock

Answer to Solved The compressibility factor, Z, can be thought of as a
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect

For compressibility factor, Z, which of the following is /are correct?

Modeling analysis of the temperature profile and trapped annular pressure induced by thermal-expanded liquid in a deep gas well - Frontiers
Gas compressibility factor Z: Ideal gas vs Real gas
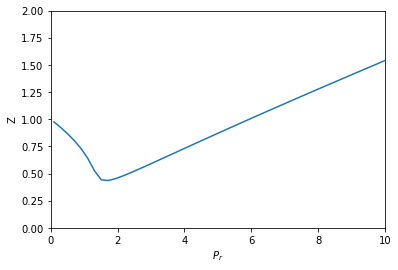
Compressibility factor variation from the van der Waals equation by three different approaches

Solved The compressibility factor, Z, can be thought of as a

The compressibility factor of a gas is defined as Z=P V / R T. The compressibility factor of idea

Gas Compressibility - an overview

If assertion is true but reason is false.

Compressibility factor (gases) - Knowino

Compressibility factor (z): real gases deviate from ideal behav-Turito

Compressibility factor - Wikipedia

Gas Compressibility - an overview

Thermodynamics: An Engineering Approach - 5th Edition - Part II by 黑傑克 - Issuu

At critical temperature, pressure and volume. The compressibility factor (Z) is 2
