Why do pressure and temperature increase during the compression of
$ 9.00 · 4.6 (664) · In stock

The energy added as work during the compression of a gas leads to an increase in pressure and temperature. Learn more about this in this article.

Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas
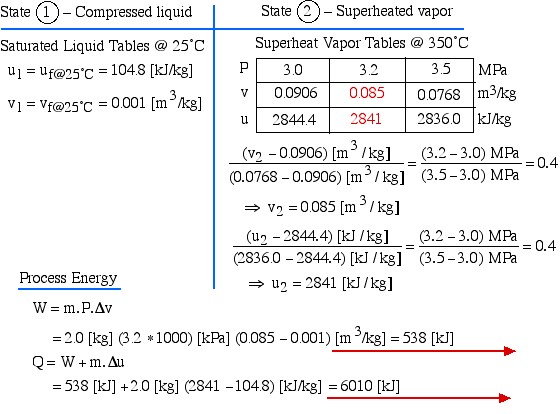
Chapter 2: The First Law of Thermodynamics for Closed Systems
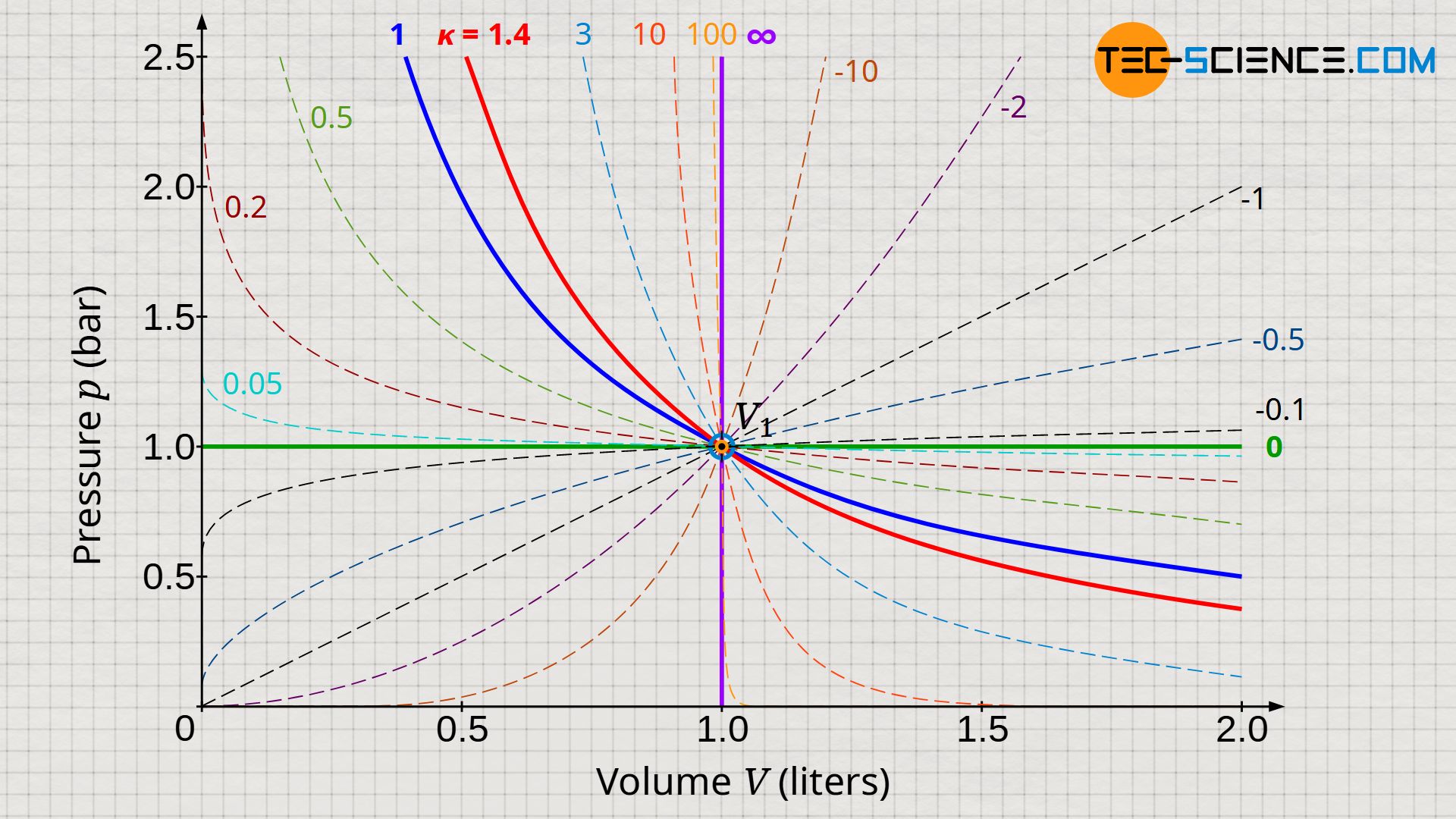
Thermodynamic processes in closed systems Archive - tec-science

Thermodynamics Archive - tec-science

Boyle's law, Definition, Equation, & Facts

Lesson 9 COMPRESSION PROCESSES Apply the ideal gas laws to SOLVE

Spray cooling technique in liquid piston gas compression and impact of air dissolution on efficiency evaluation at different pressure levels - ScienceDirect

Moisture In The Compressor - Cylinder Training Services
Why H2 gas does not show the Joule-Thomson effect?
