Five Common Mistakes Submitting a Premarket Notification
$ 14.99 · 5 (408) · In stock

How you can avoid the most common errors made when submitting a 510(k), the “premarket notification,” with simple measures

Five Common Mistakes Submitting a Premarket Notification
Mock FDA 510(k) Filing

510(k) Pre-Market Notification Project
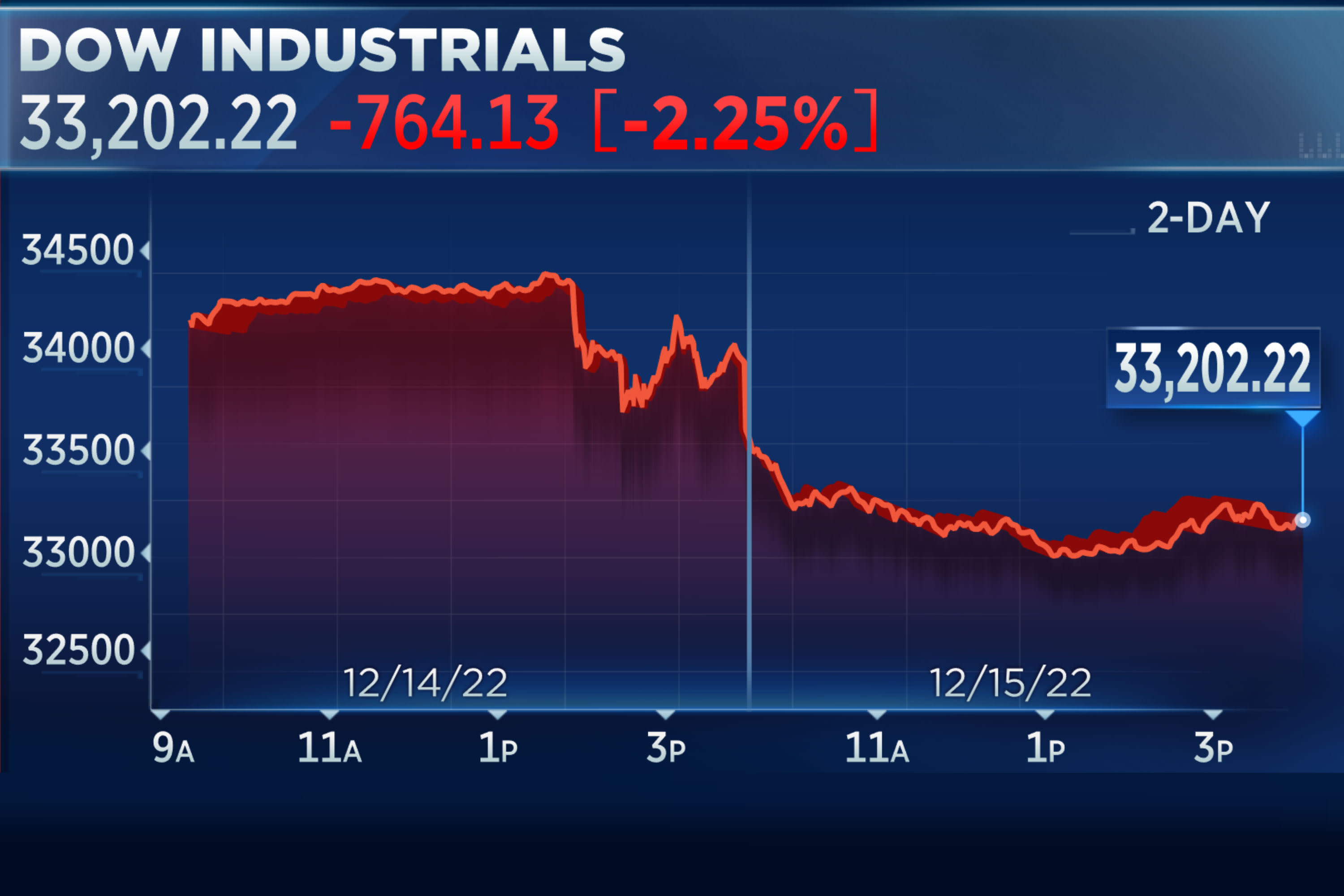
Dow closes out its worst day in three months, falls more than 700 points as recession fears grow

Avoiding Misbranding: Words Matter When Describing the Regulatory Status of 510(k) Cleared Devices and Registered Device Establishments - Life Sciences Perspectives

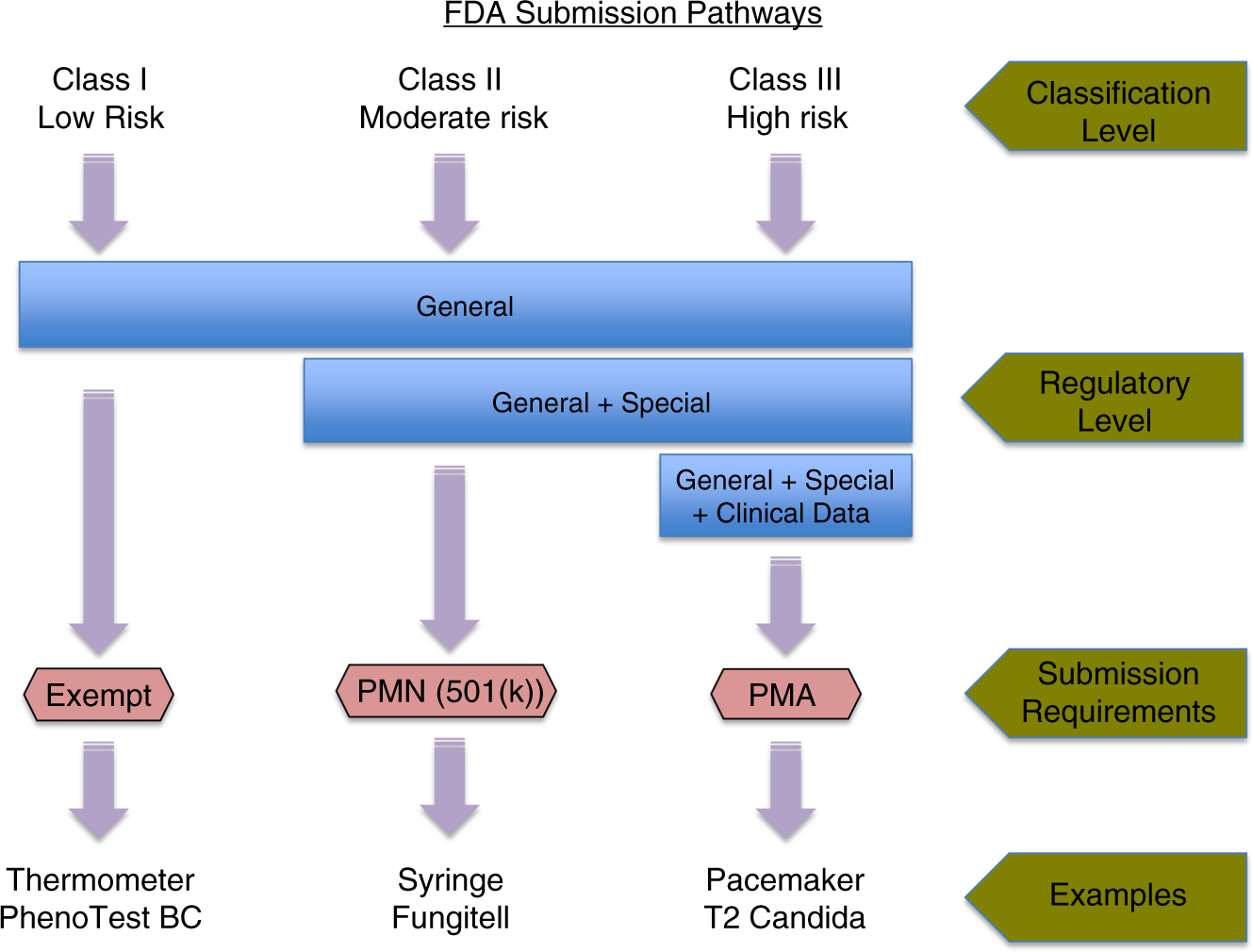
Premarket Notification The 510(k) Process
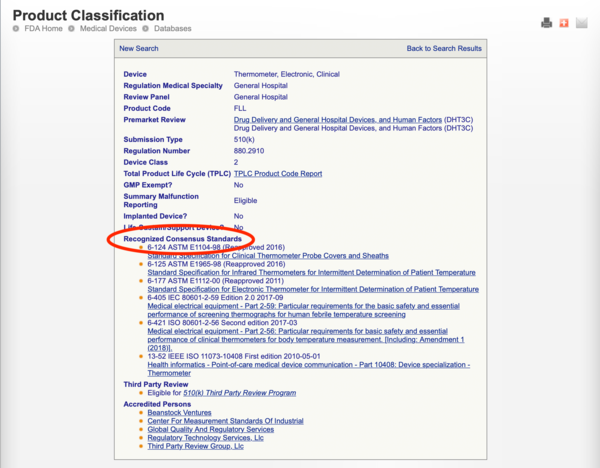
Five Common Mistakes Submitting a Premarket Notification
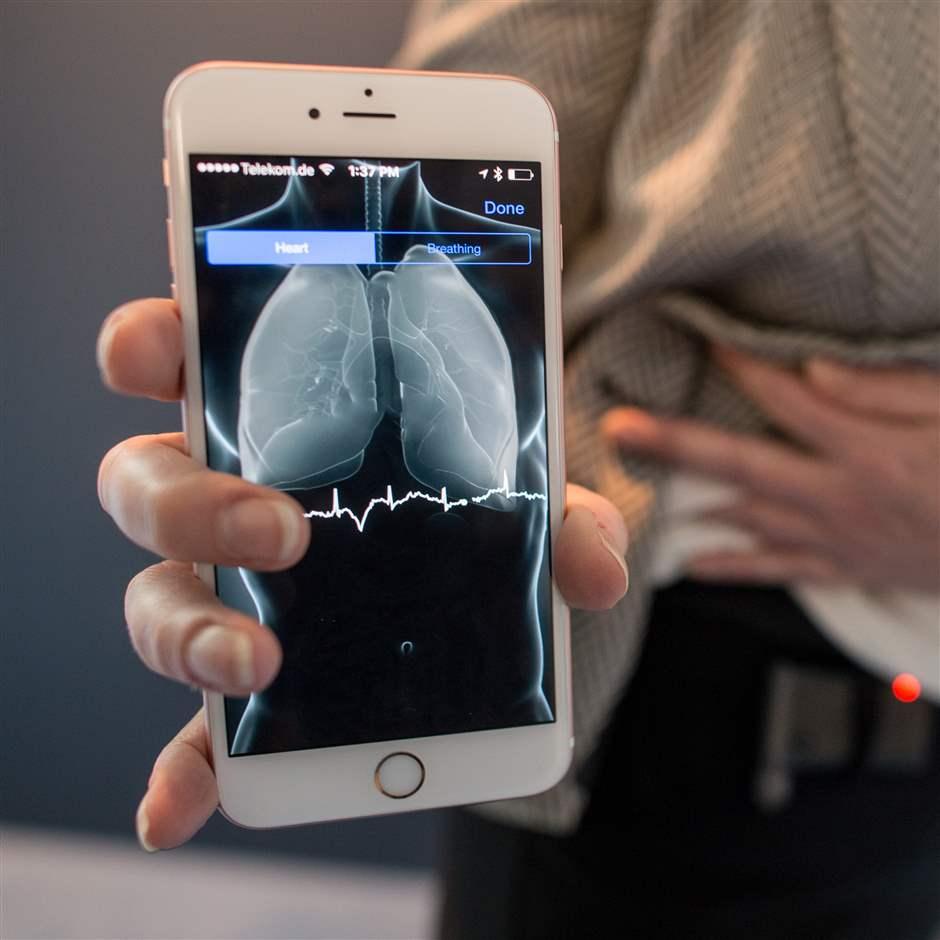
How FDA Regulates Artificial Intelligence in Medical Products

FDA 510(k) Submission: A Step-By-Step Guide On How To Prepare Yours
Molecular diagnostics in medical mycology

Five Things You Need to Know to Start Your Day: Americas - Bloomberg

A Regulatory Perspective FDA Final Guidance For Design Changes Requiring New 510(k) Submissions
