Solved] Why is the compressibility factor less than 1 at most
$ 9.00 · 5 (280) · In stock
Answer to Why is the compressibility factor less than 1 at most conditions?

Compressibility factor (z): real gases deviate from ideal behav-Turito

Non-Ideal Gas Behavior Chemistry: Atoms First

Qus.1 - 1 mole of sulphur dioxide occupies a volume of 350 ml at
PCB Substrates: Knowing PCB Dielectric Materials
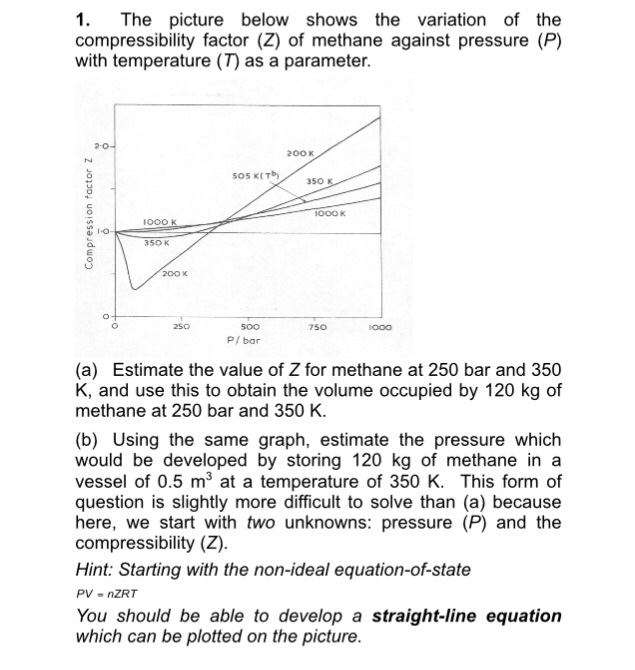
Solved 1. The picture below shows the variation of the

3.2 Real gas and compressibility factor – Introduction to
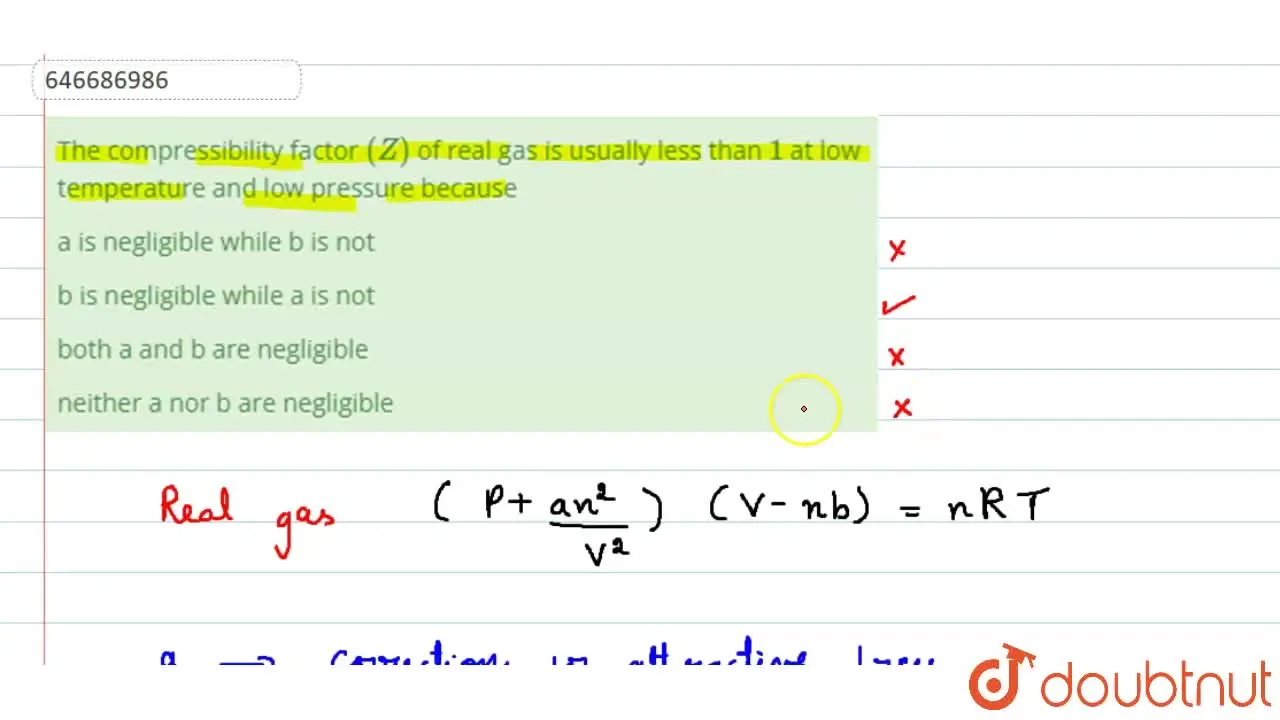
The compressibility factor (Z) of real gas is usually less than 1 at l
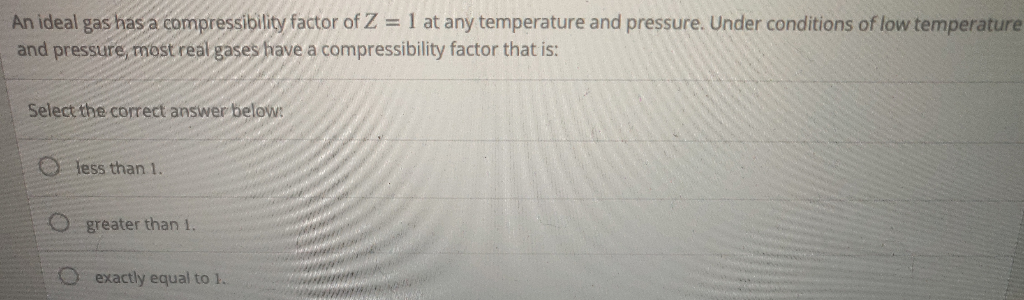
Solved An ideal gas has a compressibility factor of Z = 1 at
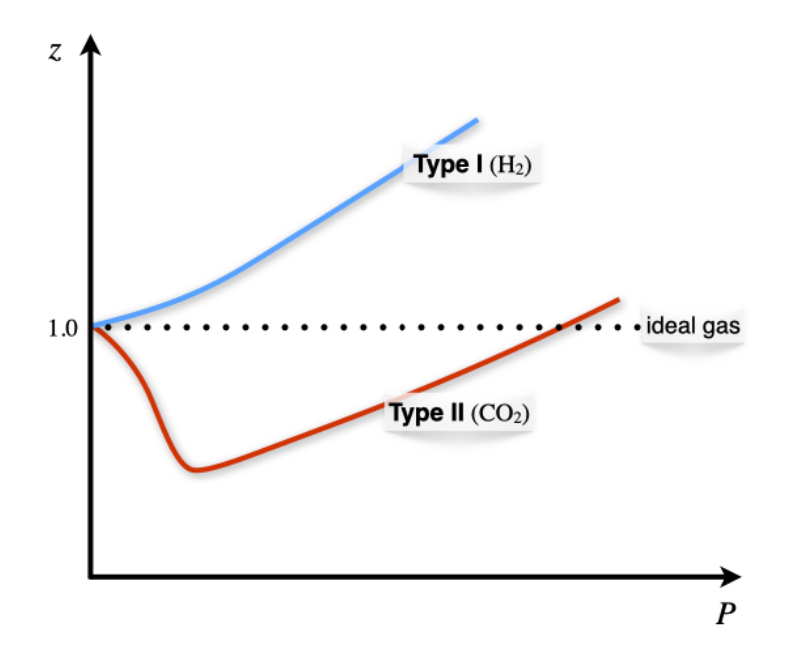
11.3: Critical Phenomena - Chemistry LibreTexts

3.2 Real gas and compressibility factor – Introduction to
Solved The plot below shows how compressibility factor (Z)