Solved 14. The latent heat of vaporization of isopropyl
$ 5.99 · 5 (124) · In stock

Answer to Solved 14. The latent heat of vaporization of isopropyl

SOLVED: Determine the total amount of heat released (in kJ) when 485 g of isopropyl alcohol (C3H8O) are cooled from the gas state at 100 °C to liquid at -42.0 °C. The

Comparison of the flash point prediction curves with experimental data
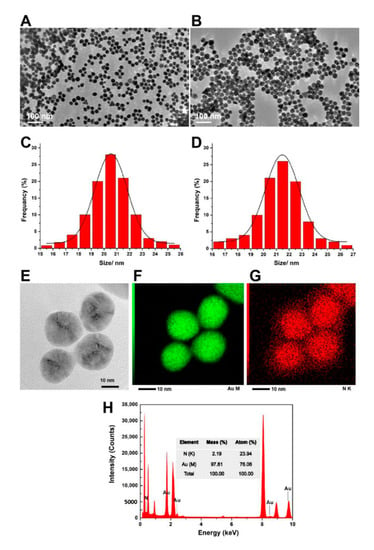
Materials, Free Full-Text

Energies, Free Full-Text
Solved 14. The latent heat of vaporization of isopropyl


A Resource utilization method for volatile organic compounds emission from the semiconductor industry: Selective catalytic oxidation of isopropanol to acetone Over Au/α-Fe2O3 nanosheets - ScienceDirect

SOLVED: Isopropyl alcohol has a heat of vaporization of 3.99 * 10^3 mol^-1 and a boiling point of 82.30 °C at 1.000 atm. Using the Clausius-Clapeyron equation, calculate the vapor pressure of

Physical properties of ethanol and isopropanol
Solved Suppose that 1.05 g of rubbing alcohol (C3H8O)
Isopropyl Alcohol, (CH3)2CHOH