SOLVED: A 25 gram piece of hot metal at 97°C is plunged into a 35 gram cup of cool water at 19°C. The metal gives up its heat to the water until
$ 6.99 · 4.9 (165) · In stock
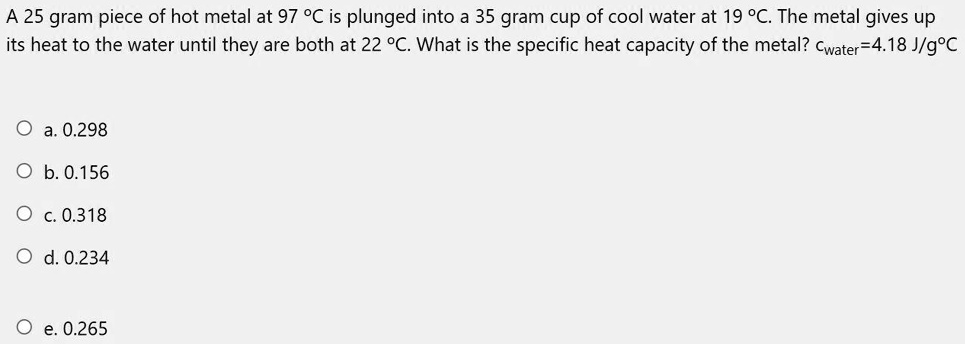
VIDEO ANSWER: in this question, there is a cup in this cup there is a water. The specific head of the water is given us 4.18 June program, degree seven degree. The water is at 19°C. The 25 g Piece of Hot Metal. This is very hot metal. The temperature
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.
A chemist heats 37.35 g of lead to 67.71 °C , then places the metal sample in the cup of water shown in the

What to Know About the Petrochemical Industry: Q&A - Moms Clean Air Force
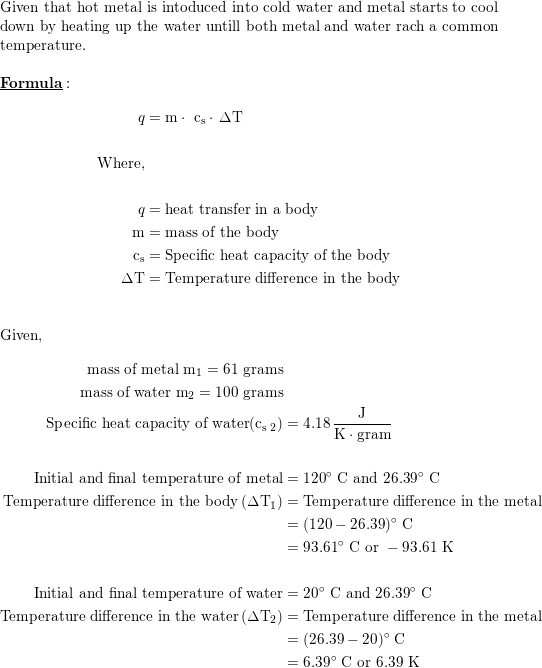
Suppose 61.0 g hot metal, which is initially at 120.0°C, is
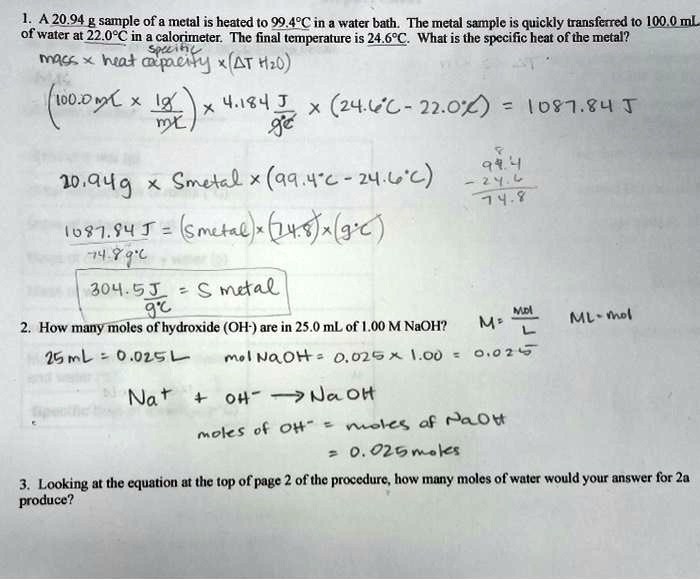
SOLVED: A 20.94 g sample of a metal is heated to 99.49°C in a water bath. The metal sample is quickly transferred to 100.0 mL of water at 22.0°C in a calorimeter.
Solved 2. A 12.9 gram sample of an unknown metal at 26.5∘C

This Peach Cobbler Smoothie in Yakima is Perfect for This Time of Year

Chapter 7 Thermochemistry - Pearson Canada

NIPS Food Production Manual by gorachand mitra - Issuu

meteorology demystified
DRAW Blog DRAW: Data Rescue Archives & Weather

HowStuffWorks Newsletter Quiz
Solved 7. A hot metal with mass 16.33 grams is transferred
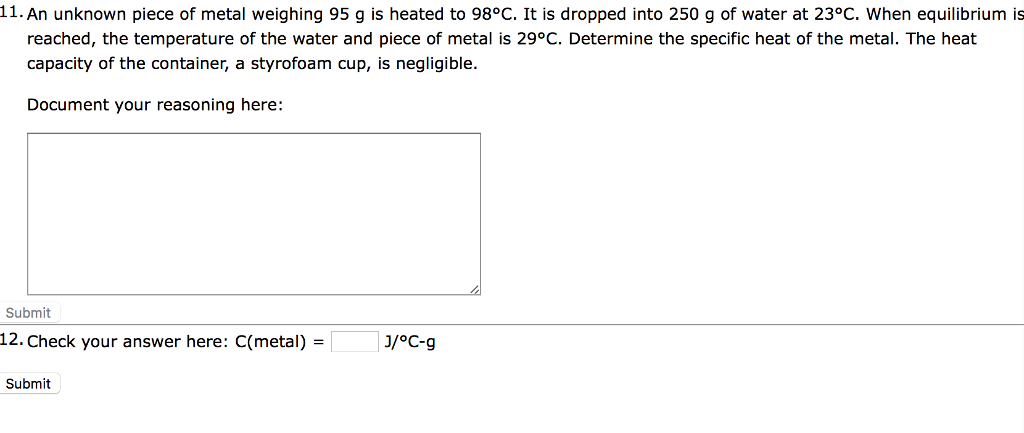
Solved 11. An unknown piece of metal weighing 95 g is heated
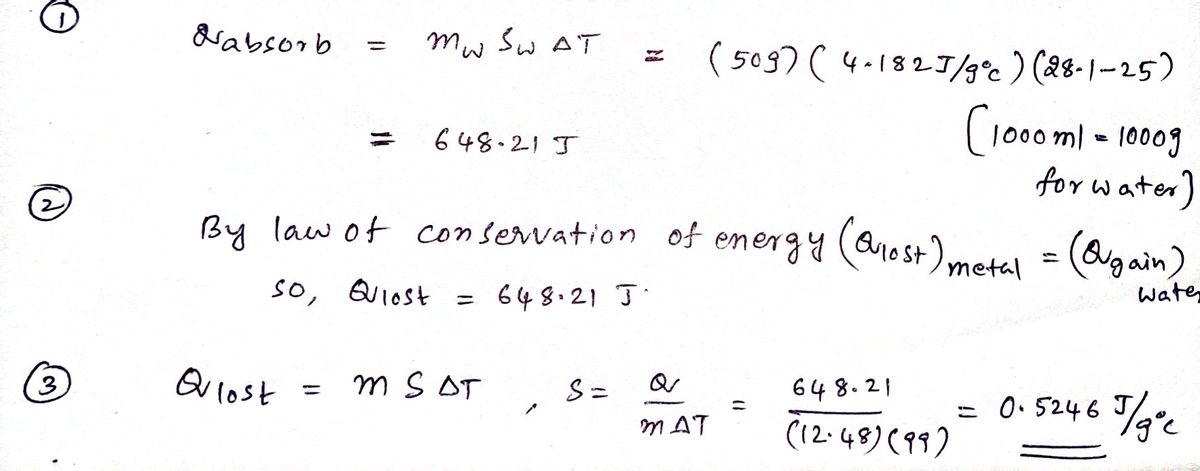
Answered: A 12.48 g sample of an unknown metal,…

Your Turn! Which of the following represents a decrease in the potential energy of the system? A book is raised six feet above the floor A ball rolls downhill. - ppt download

