Pharmaceuticals, Free Full-Text
$ 34.99 · 4.9 (298) · In stock
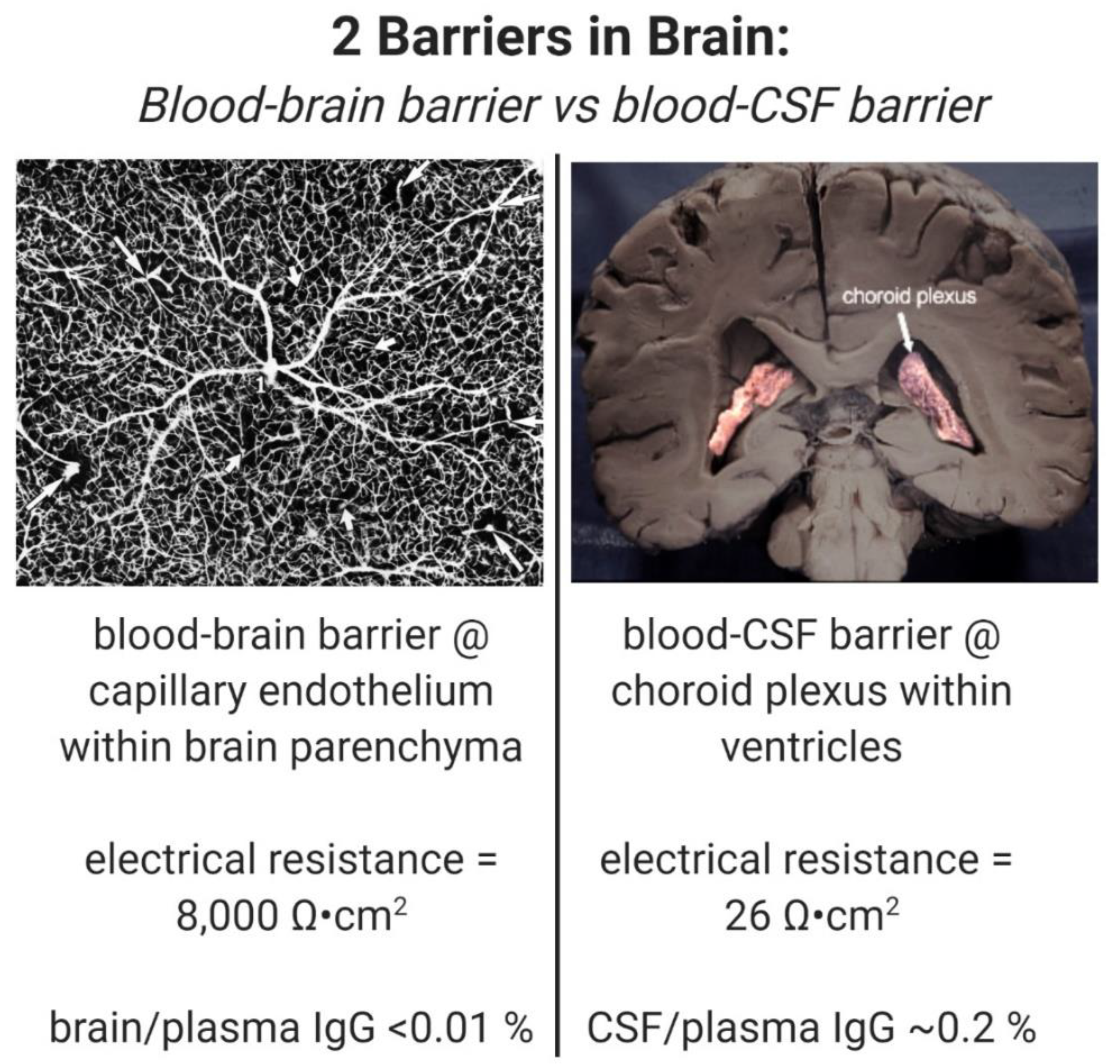
Despite the enormity of the societal and health burdens caused by Alzheimer’s disease (AD), there have been no FDA approvals for new therapeutics for AD since 2003. This profound lack of progress in treatment of AD is due to dual problems, both related to the blood–brain barrier (BBB). First, 98% of small molecule drugs do not cross the BBB, and ~100% of biologic drugs do not cross the BBB, so BBB drug delivery technology is needed in AD drug development. Second, the pharmaceutical industry has not developed BBB drug delivery technology, which would enable industry to invent new therapeutics for AD that actually penetrate into brain parenchyma from blood. In 2020, less than 1% of all AD drug development projects use a BBB drug delivery technology. The pathogenesis of AD involves chronic neuro-inflammation, the progressive deposition of insoluble amyloid-beta or tau aggregates, and neural degeneration. New drugs that both attack these multiple sites in AD, and that have been coupled with BBB drug delivery technology, can lead to new and effective treatments of this serious disorder.

MXenes and MXene-based materials for removal of pharmaceutical compounds from wastewater: Critical review - ScienceDirect
Pharmaceutical Technology Drug Development News & Views Updated DailyHome - Pharmaceutical Technology
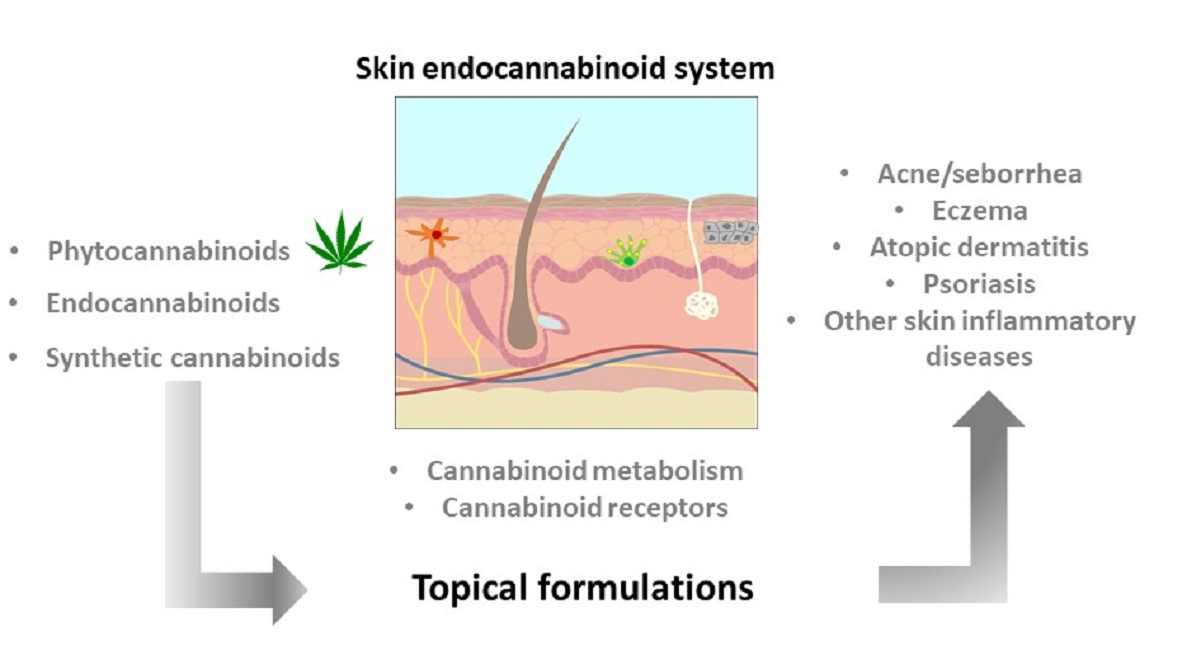
Pharmaceuticals, Free Full-Text

Infographic Templates & Designs - Venngage
Pakistan Pharma Technical Alliance on LinkedIn: Dear all friends PPTA is going to conduct free webinar to update the…
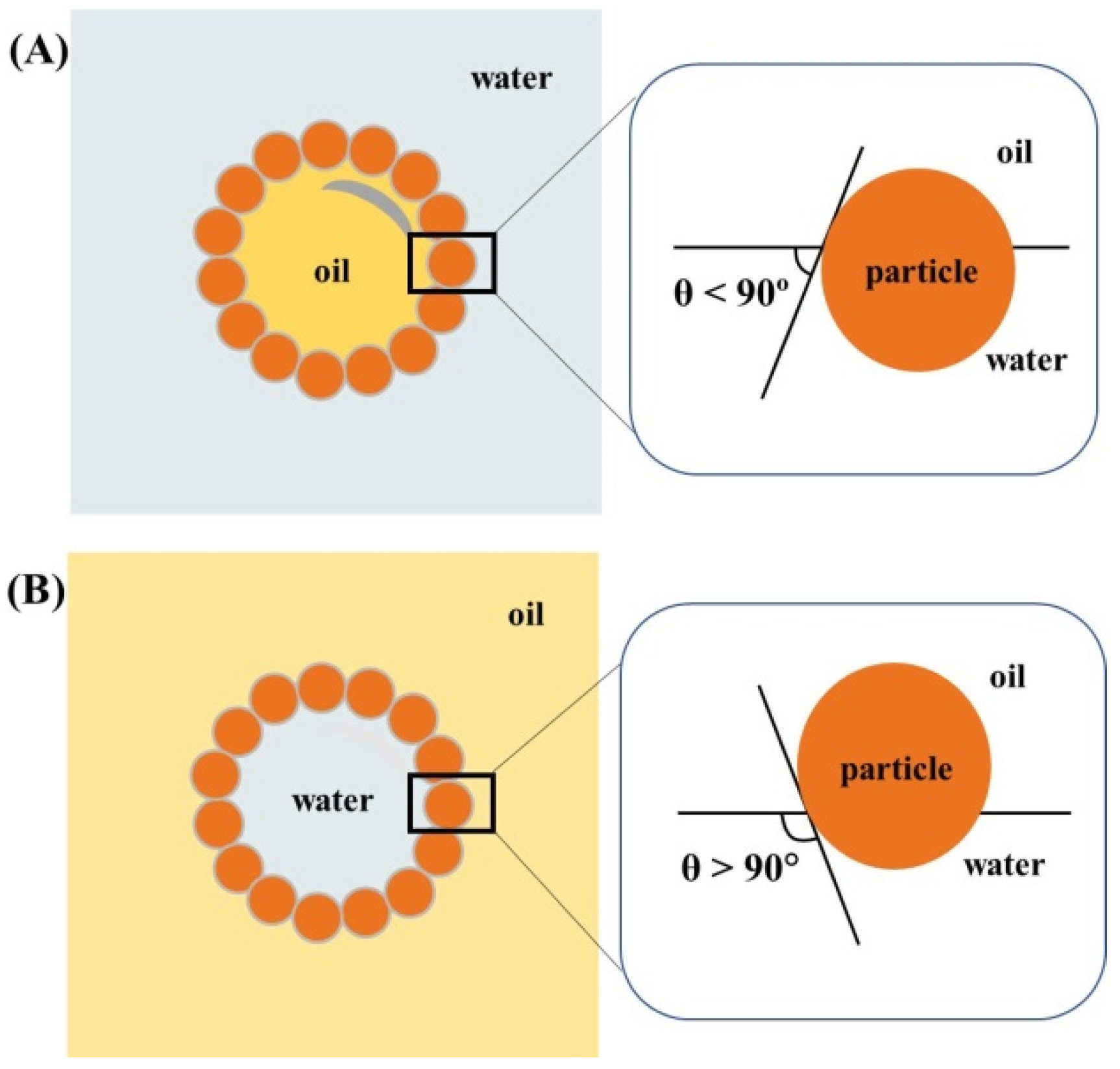
Pharmaceuticals, Free Full-Text
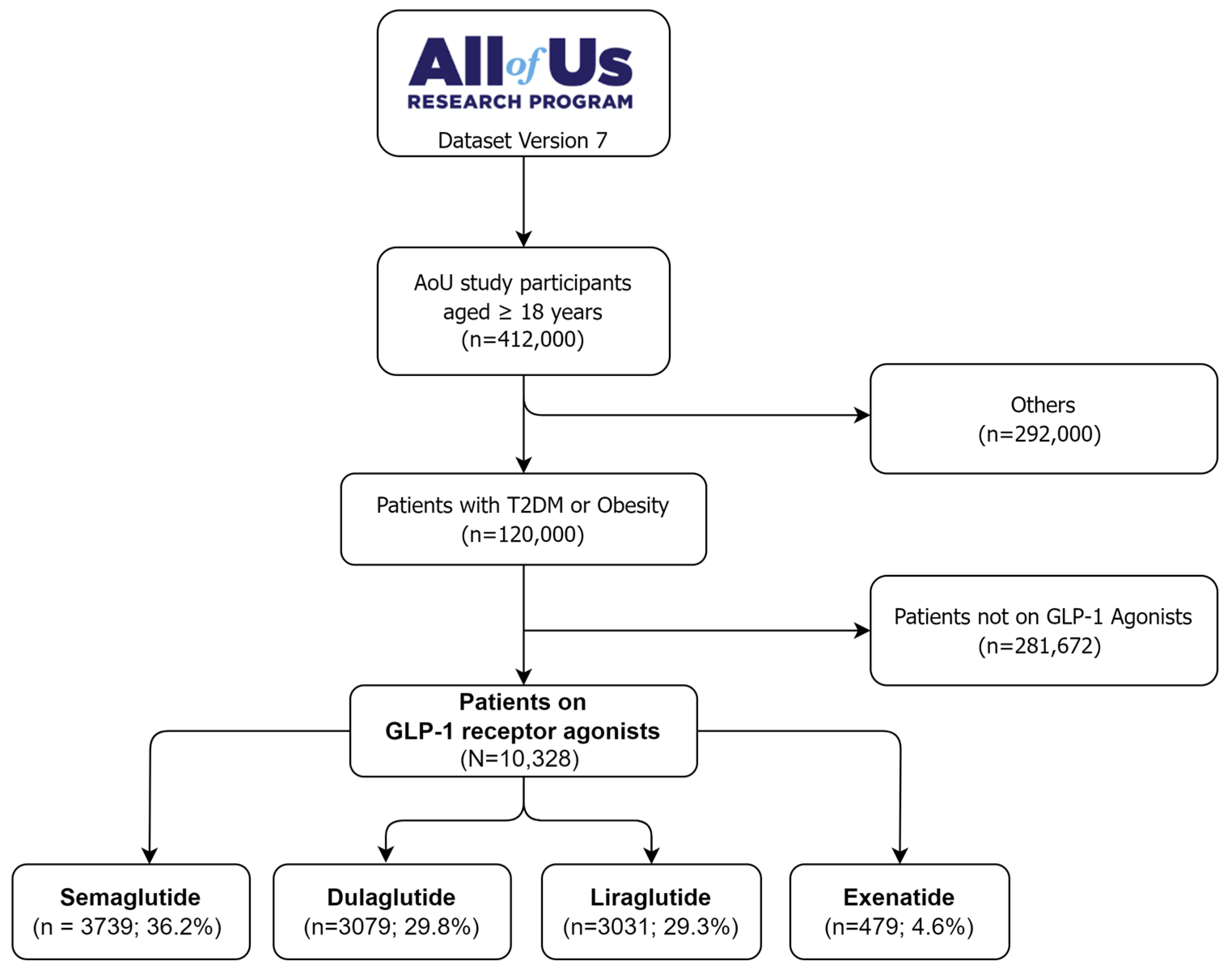
Pharmaceuticals, Free Full-Text
SMS Pharmaceuticals Ltd. – An Integrated Pharma Company with world class Bulk drugs

Pharmaceuticals Free Full-Text Effects Of, 56% OFF

PDF) Latin American pharmaceutical overview
TCI offers free birth control in Tulsa County
