Medtronic Insulin Pump Devices Recalled Due to Serious Risks
$ 29.00 · 4.6 (662) · In stock
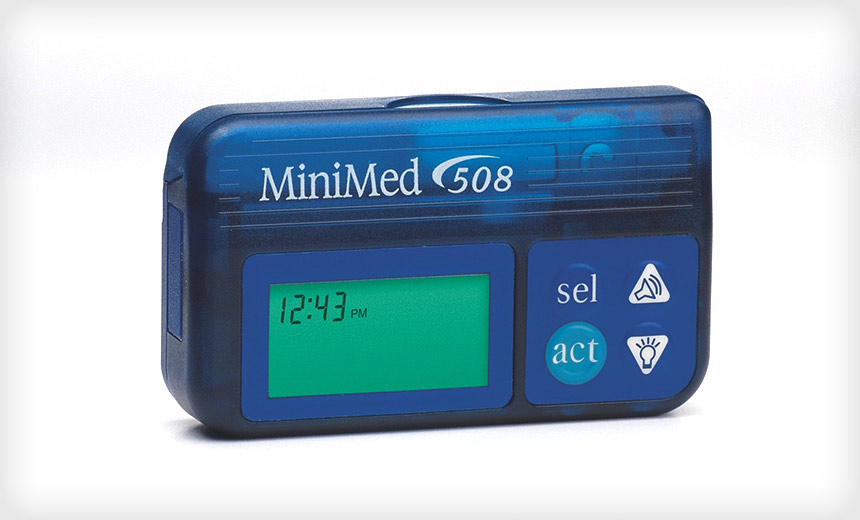
The Food and Drug Administration on Tuesday issued a warning notifying patients that medical device maker Medtronic has expanded a recall of remote controllers for

The MiniMed™ 630G System
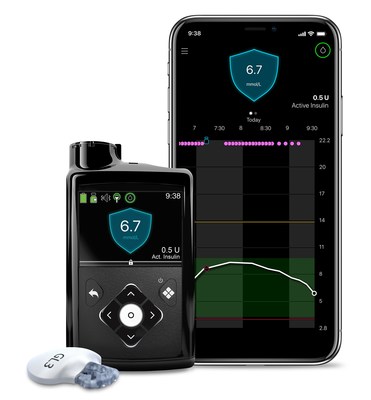
Medtronic continuous glucose monitoring to be reimbursed for
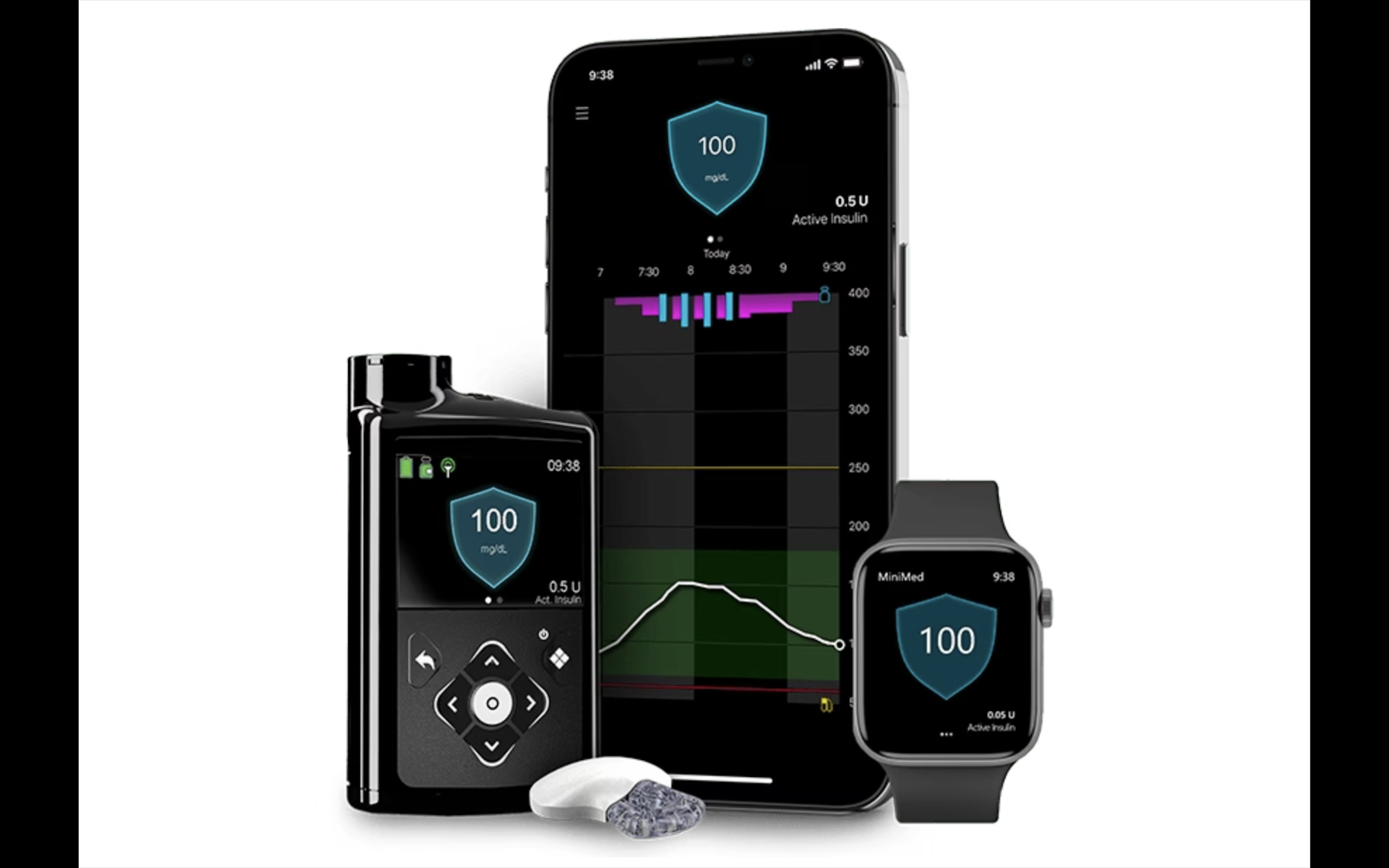
Medtronic's new insulin pump gains FDA approval

Medtronic Insulin Pump Litigation

Medtronic Recalls Insulin Pumps Over Concerns For Hacking Risk

Page 312 - Latest breaking news articles on data security breach

FDA Recalls MiniMed Insulin Pumps Due to Injury and Death

Diabetes and the benefits, risks of personal health on the internet

Medtronic's new insulin pump gains FDA approval
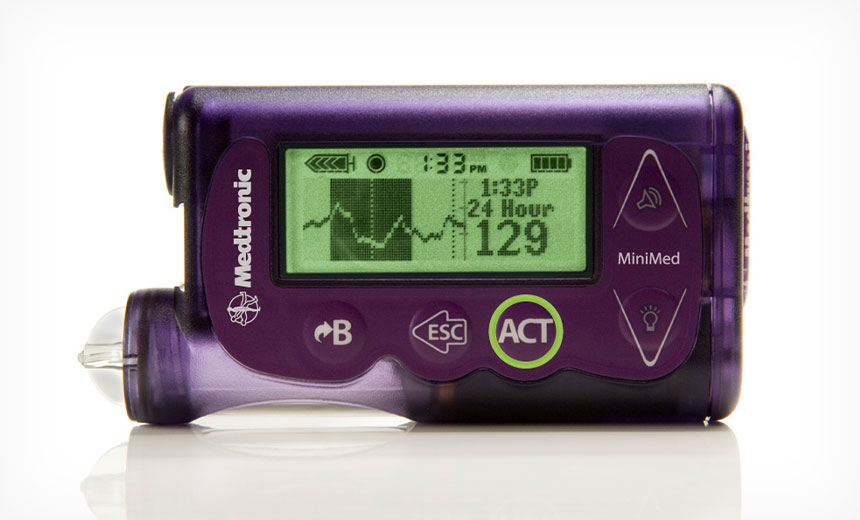
Certain Insulin Pumps Recalled Due to Cybersecurity Issues

Medtronic Insulin Pump Devices Recalled Due to Serious Risks