Medical device regulations, classification & submissions
$ 14.99 · 4.9 (148) · In stock

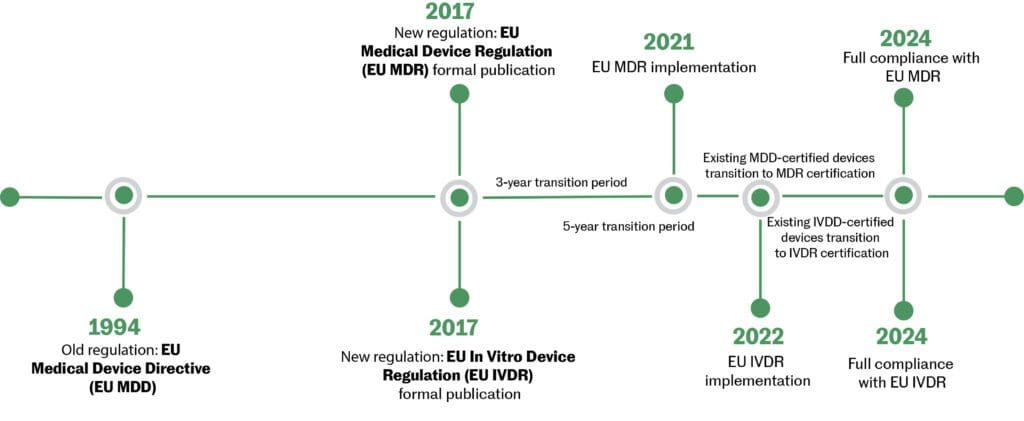
Medical device regulations vary in Canada, the U.S. & the EU. Risk-based classification systems determine data requirements for regulatory oversight for medical devices. MaRS

Medical device regulations, classification & submissions

Medical device regulations, classification & submissions

Medical device regulations, classification & submissions

Medical device regulations, classification & submissions
Medical device regulations, classification & submissions