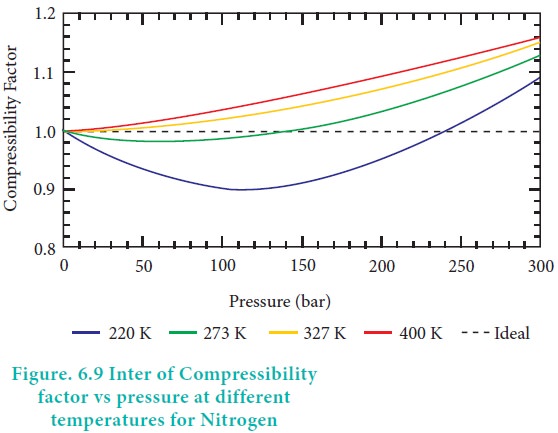
In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure
$ 7.99 · 4.9 (271) · In stock

In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure
In the following compressibility factor Z vs pressure graph at 300 K- the compressibility of CH 4 at pressure -200 bar deviates from ideal behaviourA- The molar volume of CH 4 is less than its molar volume in the ideal stateB- The molar volume of CH 4 is same as that in its ideal stateC- Intermolecular interactions between CH 4 molecules decresasesD- The molar volume of CH 4 is more than its molar volume in the ideal state

Compressibility factor - Wikipedia

Energies, Free Full-Text

The graph of compressibility factor (Z) vs. P for one mole of a
Is z (compressibility factor) vs P (pressure) graph drawn by

Real Gas: Difference, Examples and Factors
Compressibility factor Z - Gaseous State

plotting - How to plot Compressibility factor Z vs Pressure P using ParametricPlot? - Mathematica Stack Exchange

e Compressibility factor (Z) for hydrogen WRT pressure and
Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora

1.1: Thermodynamic Variables and Equations of State - Chemistry LibreTexts
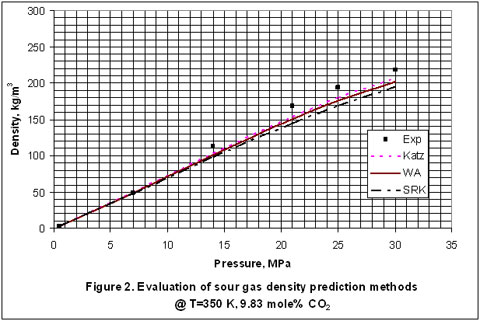
How good are the shortcut methods for sour gas density calculations?