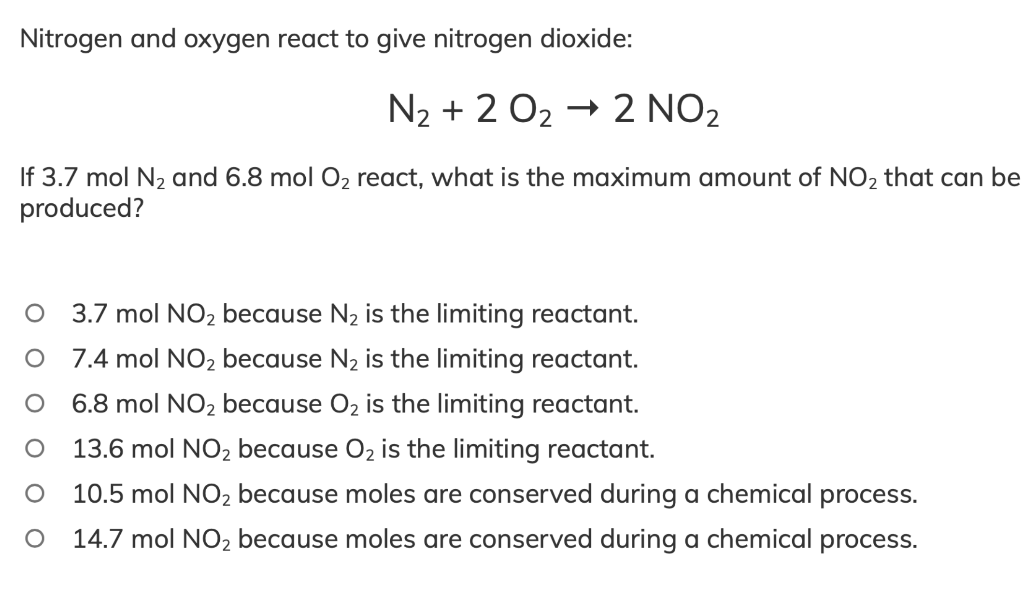42g of N₂ react with excess of O₂ to produce NO. Amount of NO
$ 17.99 · 4.8 (600) · In stock

Share your videos with friends, family, and the world

Limiting Reaction Calculations Practice Flashcards

Percent Yield Formula, How to Calculate Yield - Lesson

Answered: Consider the balanced reaction of…
Solved Nitrogen and oxygen react to give nitrogen dioxide
If 25 grams of CO reacted with 6.00 grams of H2, which is the limiting reactant and theoretically yield of CH3OH? - Quora

ChemHelp: a place to ask questions about chemistry

Answered: if 24.0g of NO and 13.8g of O2 are used…
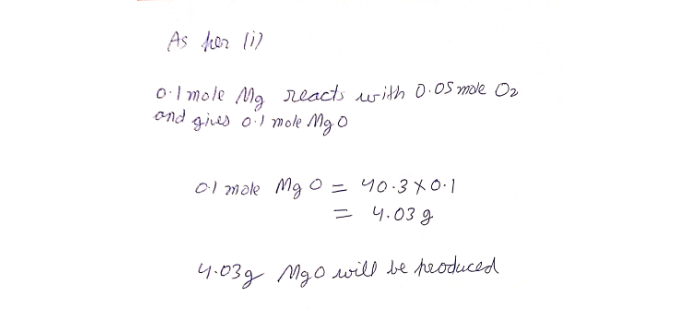
Answered: Suppose 2.43 g of magnesium is reacted…

Solved 21. Consider the following chemical reaction: N2+ O2

27 g Al reacts completely with how many grams Oxygen.

Empirical formula of a hydrocarbon having 80% C and 20% of hydrogen is a.CH b.CH3 c.CH2 d.CH4 MDCAT

stoy-key-ahm-e-tree) - ppt download
16433-96-8, 1-Ethynyl-2-nitrobenzene