13 14 15 16 17 18 19 20 21 22 23 2425 26 27 28 29 30 31 32 40% of a racem..
$ 19.00 · 4.5 (291) · In stock

Solution For 13 14 15 16 17 18 19 20 21 22 23 2425 26 27 28 29 30 31 32 40% of a racemic mixture of 0.2 moles of N2 and 0.6 moles of H2 react to give NH3 according to the equation, N2( g)+H2( g)
13 14 15 16 17 18 19 20 21 22 23 2425 26 27 28 29 30 31 32 40% of a racemic mixture of 0.2 moles of N2 and 0.6 moles of H2 react to give NH3 according to the equation, N2( g)+H2( g) ₹ pressure. Then the ratio of the final volume to the initial volume of gases is:
Video solution 1: 13 14 15 16 17 18 19 20 21 22 23 2425 26 27 28 29 30 31 32 40% of a racemic mixture of 0.2 moles of N2 and 0.6 moles of H2 react to give NH3 according to the equation, N2( g)+H2( g) ₹ pressure. Then the ratio of the final volume to the initial volume of gases is

1 567 16 17 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38

Comorbidity, misdiagnoses, and the diagnostic odyssey in patients
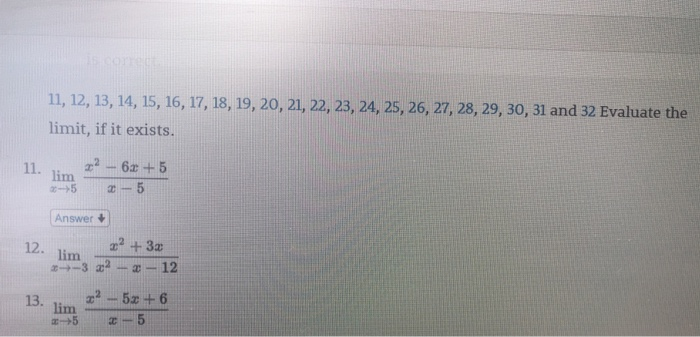
Solved 1,12, 13,14, 15, 16, 17, 18, 19, 20, 21,22, 23, 24
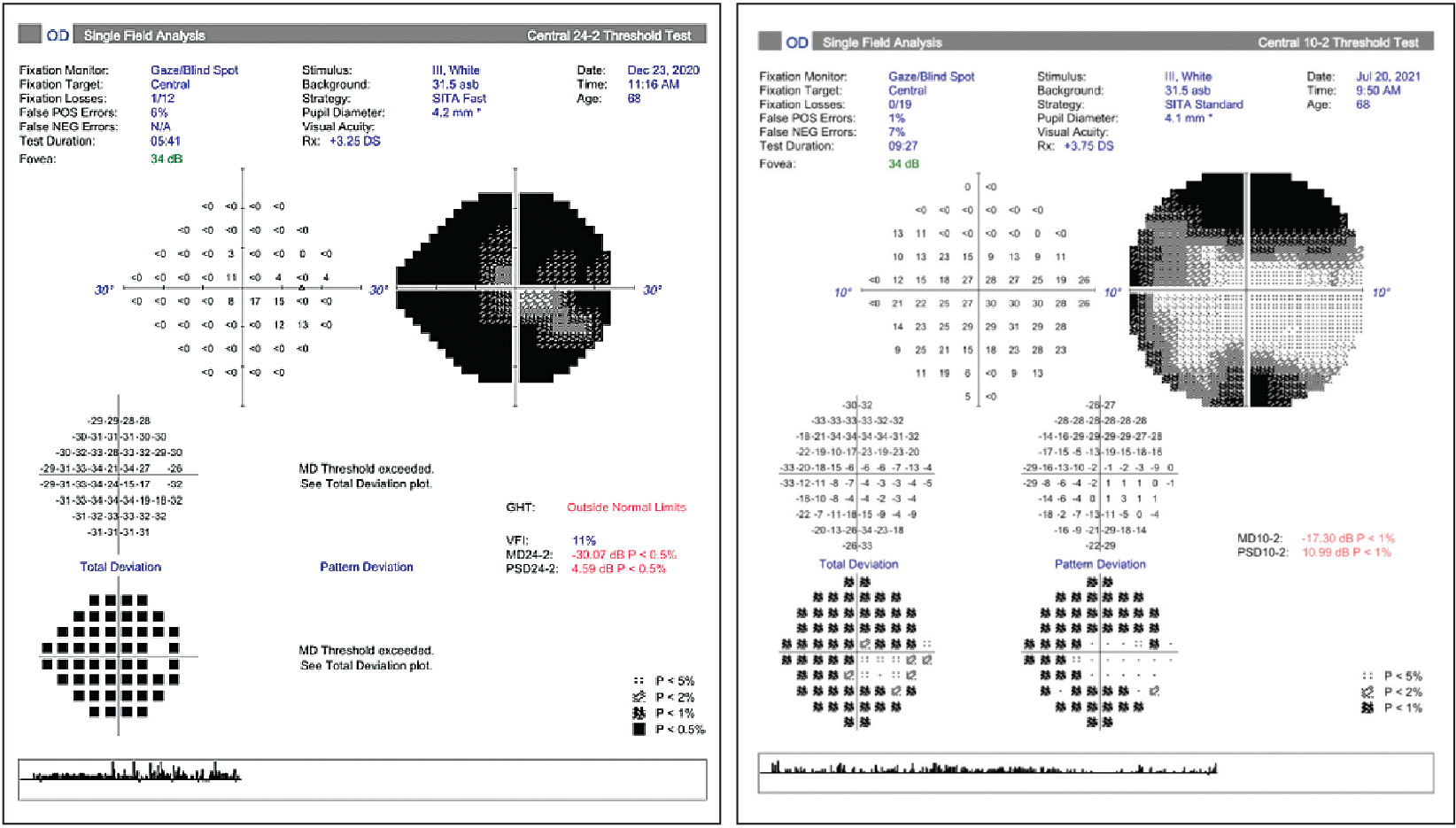
Prepping for the Glaucoma Grind

Aquatics - Spring 2020 by Portland Parks & Recreation - Issuu
Solved 11. 12. 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23
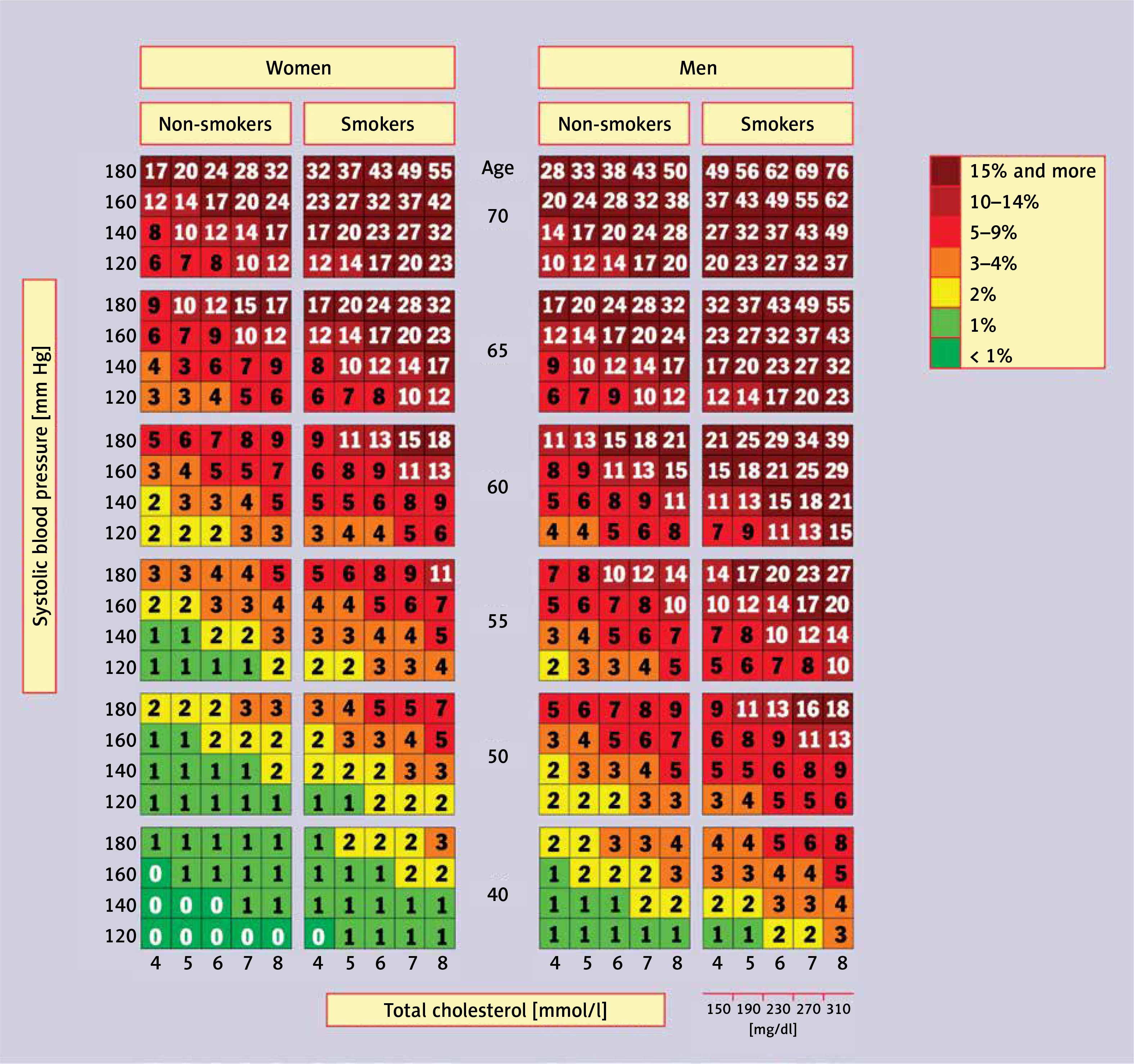
PoLA/CFPiP/PCS/PSLD/PSD/PSH guidelines on diagnosis and therapy of
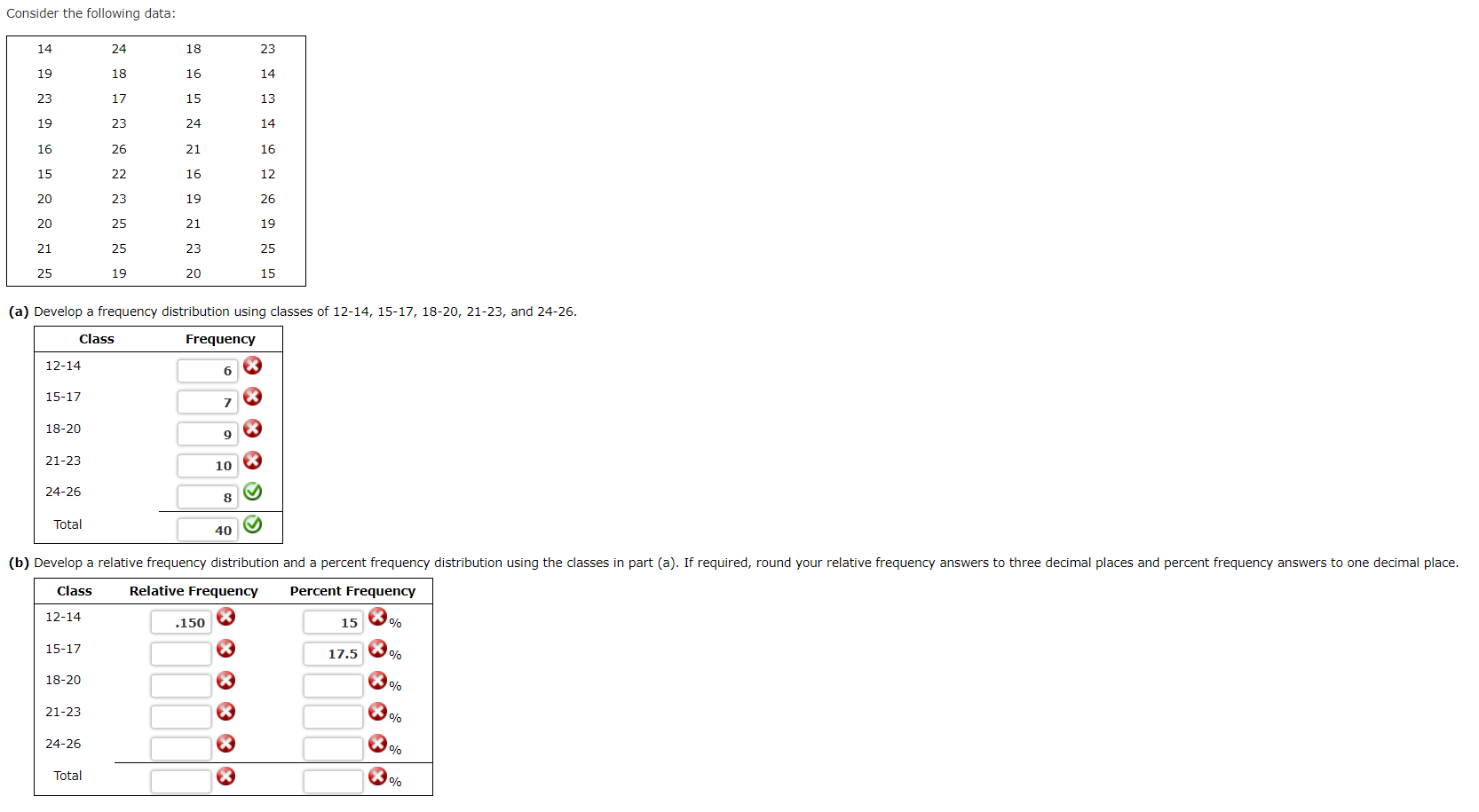
Solved Consider the following data: 14 24 18 23 19 18 16 14
PPT - dos PowerPoint Presentation, free download - ID:4486095