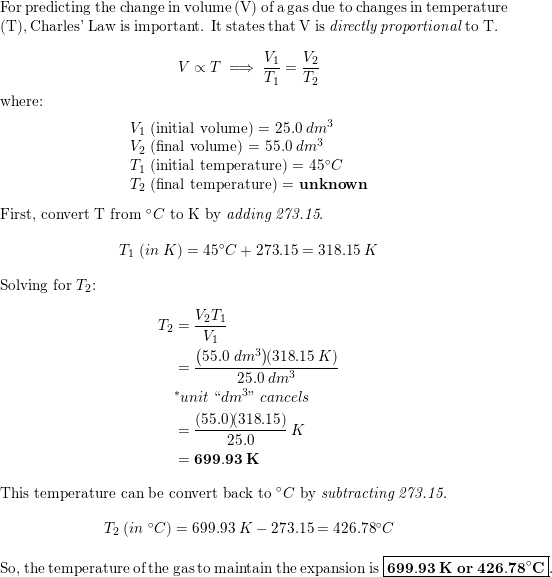
A piston containing $25.0 \mathrm{dm}^{3}$ of gas at $45^{\c
$ 18.99 · 4.7 (313) · In stock

Estimating the Jet Power of Mrk 231 during the 2017–2018 Flare - IOPscience

Homework 15 Gas Laws.pdf - Homework 15 - Gas Laws 1. A sample of nitrogen gas in a 1.75 L container exerts pressure of 1.35 atm at 25 °C. What is
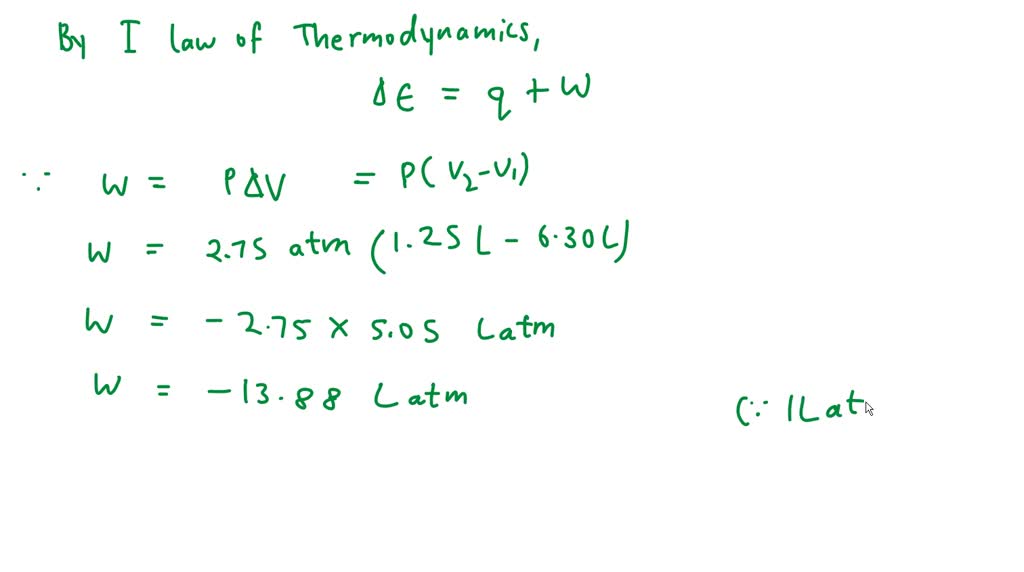
SOLVED: A piston containing a gas is compressed from a volume of 6.30 L to 1.25 L against a constant pressure of 2.75 atm. while there is a heat loss by the
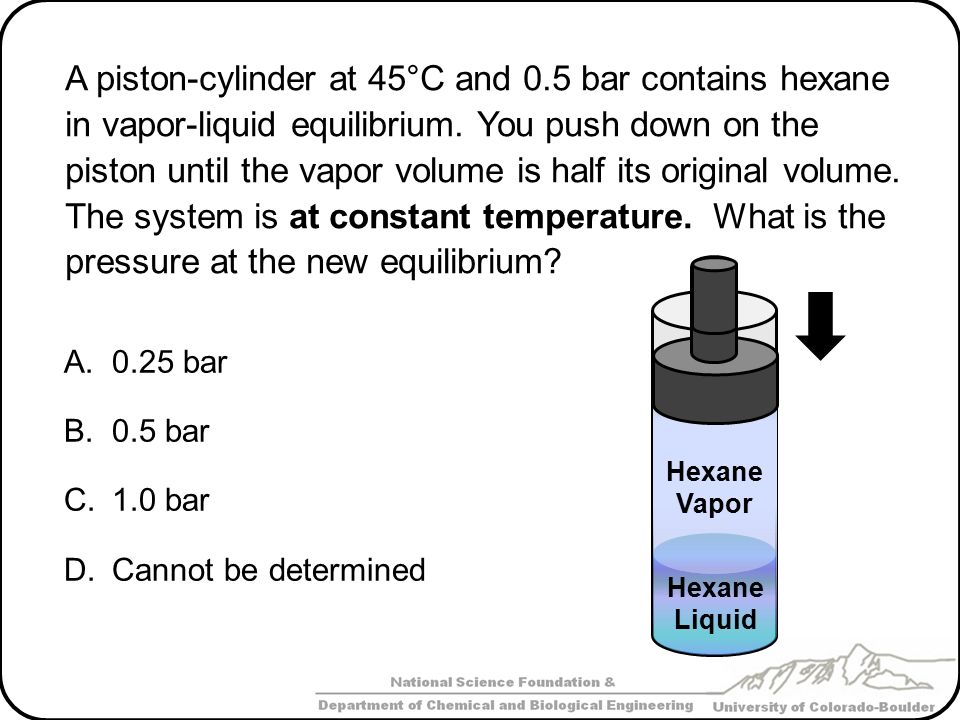
Phase Equilibrium: Single Condensable Component Part 1 - ppt video online download

combinedGasLawAndIdealGasLawWkst.docx - Worksheet: Combined Gas Law and Ideal Gas Law Name 1. A 952 cm3 container of gas is exerting a pressure of 108


Answered: The gas in a 250.0 mL piston…

xw27/scibench · Datasets at Hugging Face

Estimating the Jet Power of Mrk 231 during the 2017–2018 Flare - IOPscience

Estimating the Jet Power of Mrk 231 during the 2017–2018 Flare - IOPscience

Solved Problem 7.13 Nitrogen Piston A piston-fitted cylinder

Chapter 2, Heat, Work, Internal Energy, Enthalpy, and the First Law of Thermodynamics Video Solutions, Physical Chemistry
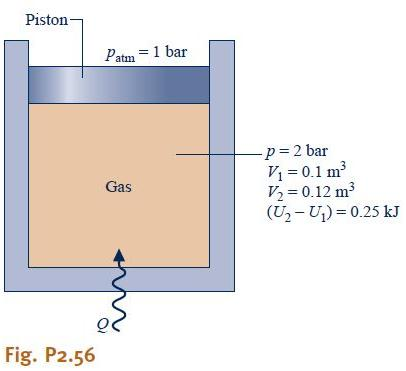
Solved Question